How Insulin Gene Mutations Contribute to MODY Diabetes?
- admin
- December 2, 2024
- 6:39 pm
- No Comments
Mutations in the insulin gene (INS) are a recognized cause of Maturity-Onset Diabetes of the Young (MODY), a rare monogenic form of diabetes.
Unlike Type 1 or Type 2 diabetes, MODY is caused by a single gene defect, with the INS gene being one of the rare but critical contributors.
This article by bestdietarysupplementfordiabetics.com delves into the mechanisms through which insulin gene mutations lead to MODY, examining their molecular effects, clinical presentations, and diagnostic implications.
We will also explore real-life cases, supporting studies, and genetic advancements in understanding this unique diabetes form.
Table of Contents:
- Introduction to MODY and Insulin Gene Mutations
- The Role of the Insulin Gene (INS) in Beta-Cell Function
- How INS Mutations Disrupt Insulin Production
- Clinical Manifestations of MODY Due to INS Mutations
- Real-Life Case Study: David’s MODY Diagnosis
- Diagnostic Tools for Detecting INS Gene Mutations
- Research Insights on INS Mutations and MODY
- Impact on Diabetes Management and Treatment Strategies
- FAQs on Insulin Gene Mutation & MODY
- Conclusion: Understanding the Role of INS Mutations in MODY
Introduction to MODY and Insulin Gene Mutations
MODY (Maturity-Onset Diabetes of the Young) is a monogenic form of diabetes caused by mutations in genes essential for beta-cell function.
Among these, the INS gene, responsible for encoding preproinsulin (a precursor to insulin), plays a significant role.
Mutations in the INS gene disrupt the proper folding and processing of preproinsulin, leading to misfolded proteins and endoplasmic reticulum (ER) stress in beta cells.
This results in reduced insulin production and secretion, impairing glucose regulation.
For example, mutations like INS E21K alter the structure of preproinsulin, making it prone to aggregation and beta-cell dysfunction.
These genetic disruptions manifest in early-onset diabetes, often appearing in adolescence or even infancy.
Unlike other MODY forms, INS-related MODY can mimic neonatal diabetes due to its severity.
A study in Diabetologia (2020) highlighted that INS mutations account for 1–2% of all MODY cases, emphasizing the importance of early genetic testing for proper diagnosis and management.
The Role of the Insulin Gene (INS) in Beta-Cell Function
The INS gene is located on chromosome 11 and is responsible for encoding preproinsulin, a precursor to functional insulin.
Insulin is crucial for glucose regulation, promoting cellular uptake of glucose and maintaining blood sugar balance.
In beta cells, preproinsulin undergoes post-translational modifications to form mature insulin and C-peptide, which are secreted in response to glucose stimulation.
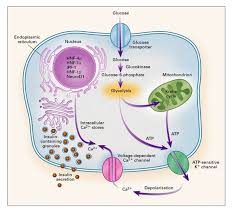
Any mutation in the INS gene can hinder this process, leading to misfolded proteins, beta-cell stress, and impaired insulin secretion.
A study in Diabetes Care (2018) highlighted the role of INS mutations in triggering endoplasmic reticulum (ER) stress, causing beta-cell apoptosis and reducing insulin production capacity.
How INS Mutations Disrupt Insulin Production?
Mutations in the INS gene, which encodes preproinsulin, lead to significant disruptions in insulin production and secretion through several molecular pathways:
Misfolded Insulin Proteins:
Mutations in the INS gene often result in structural abnormalities in preproinsulin, the precursor of insulin.
These abnormalities cause the protein to misfold within the endoplasmic reticulum (ER). Misfolded proteins accumulate in the ER, activating the unfolded protein response (UPR).
While UPR is a protective mechanism, prolonged activation overwhelms cellular repair systems, leading to beta-cell stress and apoptosis. This process is a hallmark of INS-related MODY.
Impaired Beta-Cell Function:
The chronic ER stress induced by misfolded insulin proteins compromises beta-cell functionality. As beta cells struggle to manage stress, their ability to respond to elevated glucose levels is impaired, reducing insulin secretion.
This disruption is a direct cause of hyperglycemia in MODY.
Reduced Insulin Levels:
Even when some functional insulin is produced, its quantity is insufficient to maintain glucose balance. This partial insulin deficiency, compounded by beta-cell exhaustion, manifests in the early onset of diabetes.
Scientific Evidence:
Research published in Nature Genetics (2020) investigated the folding and stability of preproinsulin in INS mutations. It confirmed that specific mutations, such as INS C96Y, directly impair the structural integrity of preproinsulin, triggering UPR and beta-cell failure.
These mechanisms explain why INS mutations lead to the unique clinical presentation of MODY, characterized by early-onset hyperglycemia and a progressive decline in beta-cell function.
Clinical Manifestations of MODY Due to INS Mutations
Patients with MODY caused by INS gene mutations display a unique set of clinical characteristics, enabling differentiation from other forms of diabetes:
- Early Onset: Symptoms typically develop before the age of 25, often during adolescence or early adulthood, which sets it apart from Type 2 diabetes.
- Mild Fasting Hyperglycemia: Blood glucose levels are persistently elevated but generally not severe enough to cause immediate complications.
- Absence of Obesity: Unlike Type 2 diabetes, MODY patients are typically lean, with no association between obesity and disease onset.
- No Autoantibodies: Unlike Type 1 diabetes, patients lack autoantibodies, ruling out an autoimmune cause.
- Family History: MODY follows an autosomal dominant inheritance pattern, meaning a parent with the mutation has a 50% chance of passing it to their offspring. This familial link is often key in diagnosing MODY.
For instance, a study in Diabetologia (2018) emphasized the distinct absence of insulin resistance or obesity in MODY patients, further differentiating it from other diabetes forms.
Recognizing these features is critical for early diagnosis and appropriate management.
Real-Life Case Study: David’s MODY Diagnosis
Here is a classic case study:
Let me walk you through with this case:
Background:
David, a 19-year-old athlete, experienced persistent mild hyperglycemia during annual health check-ups. Despite leading an active lifestyle, his fasting glucose levels remained elevated.
His family history revealed that his father was diagnosed with diabetes in his late 20s but managed it with minimal medication.
Diagnosis and Insights:
After initial misdiagnosis with Type 1 diabetes, David underwent genetic testing, which identified an INS mutation.
This confirmed a diagnosis of MODY. He was treated with sulfonylureas, achieving stable blood glucose levels without insulin therapy.
David’s case illustrates the importance of recognizing genetic contributions to diabetes, particularly in non-obese individuals with a family history of early-onset diabetes.
Diagnostic Tools for Detecting INS Gene Mutations
Diagnosing MODY caused by INS gene mutations requires a comprehensive approach to distinguish it from other diabetes forms. Key diagnostic steps include:
Genetic Testing
- Techniques such as Next-Generation Sequencing (NGS) and Polymerase Chain Reaction (PCR) are used to pinpoint mutations in the INS gene.
- Genetic testing is considered the gold standard, offering definitive confirmation of MODY and guiding personalized treatment strategies.
- Biochemical Markers
- Patients with MODY due to INS mutations typically present with persistent fasting hyperglycemia, unaccompanied by insulin resistance or autoantibodies.
- This biochemical profile differentiates MODY from both Type 1 and Type 2 diabetes.
- Family History Analysis
- A thorough evaluation of family medical history often reveals autosomal dominant inheritance, with multiple generations affected by early-onset diabetes.
- Patterns of diabetes in lean individuals without insulin resistance strengthen the case for MODY.
A study published in Endocrine Reviews (2019) highlighted the pivotal role of genetic testing in improving the accuracy of MODY diagnoses.
By identifying specific gene mutations, healthcare providers can avoid unnecessary insulin therapy and instead implement treatments tailored to the patient’s genetic profile.
Early diagnosis also facilitates family-wide genetic screening, enabling proactive management for at-risk relatives.
Research Insights on INS Mutations and MODY
Several pivotal studies have expanded our understanding of how INS mutations contribute to the development of MODY diabetes:
- Diabetologia (2021): Demonstrated that INS mutations induce chronic endoplasmic reticulum (ER) stress, leading to progressive beta-cell failure. This provides a molecular explanation for reduced insulin secretion in MODY patients.
- Journal of Clinical Endocrinology & Metabolism (2018): Identified INS mutations as the cause of up to 5% of MODY cases in specific populations, highlighting the significance of genetic diversity in MODY etiology.
- Diabetes (2020): Emphasized the importance of early diagnosis and intervention, showing that timely management significantly improves long-term outcomes for MODY patients with INS mutations.
These findings illustrate the genetic complexity of MODY and underscore the necessity of integrating genetic research into clinical practice.
Understanding the unique characteristics of INS-related MODY enables precise diagnosis and effective, tailored treatments, reducing complications and enhancing patient quality of life.
Impact on Diabetes Management and Treatment Strategies
MODY resulting from INS mutations requires a unique and targeted treatment strategy, distinct from other diabetes forms:
- Sulfonylureas: Patients with INS mutations typically respond well to sulfonylureas, which enhance insulin secretion from partially functional beta cells.
- Avoidance of Insulin Therapy: Unlike Type 1 diabetes, patients with MODY generally do not require insulin therapy unless beta-cell function deteriorates significantly. Accurate diagnosis helps avoid unnecessary insulin use.
- Lifestyle Modifications: A balanced diet and regular physical activity are essential to supporting overall glucose regulation, complementing pharmacological interventions.
A study published in Diabetes Care (2018) highlighted the effectiveness of sulfonylureas in improving glycemic control in MODY patients with INS mutations, reinforcing the importance of tailored treatments.
These strategies underscore the need for accurate genetic diagnosis to prevent overtreatment or inappropriate therapy, ensuring patients achieve optimal long-term outcomes.
FAQs on Insulin Gene Mutation & MODY
Q-1: What exactly do INS (insulin gene) mutations do to beta-cells in MODY?
A-1: Many INS variants change how proinsulin folds. Misfolded proinsulin jams the endoplasmic reticulum, triggers an unfolded-protein stress response, and accelerates beta-cell burnout. The result isn’t just “less insulin made”—it’s fewer healthy beta-cells over time, so secretion drops even if glucose demands stay the same.
Q-2: Why can INS-MODY be confused with type 1 diabetes—even when antibodies are negative?
A-2: People often present young and lean with high glucose, but without islet autoantibodies. C-peptide may be measurable (not absent) early on, and fasting glucose can be near-normal with big post-meal spikes. A strong autosomal-dominant family pattern and persistently negative antibodies are the clinical breadcrumbs that point to an INS mutation rather than autoimmunity.
Q-3: How do INS mutations change the “shape” of insulin in the blood tests I already get?
A-3: Because processing is faulty, proinsulin sometimes “leaks” into circulation. You may see hyperproinsulinemia—a higher proinsulin-to-insulin (or proinsulin-to-C-peptide) ratio—despite only modest total insulin output. That skewed ratio is a clue that the problem is synthesis/processing inside the beta-cell, not just insulin resistance in the body.
Q-4: Do therapy choices differ from other MODY forms like GCK or HNF1A?
A-4: Often, yes. GCK-MODY typically needs no drug therapy; HNF1A-MODY often responds dramatically to low-dose sulfonylureas. INS-MODY is variable: some individuals do well on meal-time agents or low-dose basal insulin, others need full insulin therapy earlier because beta-cell stress limits secretory reserve. Across regimens, the shared principle is to minimize secretory strain—steady glucose targets, protein- and fiber-forward meals, and avoidance of large post-meal spikes.
Q-5: What does “inherited” look like for INS-MODY—and how should families plan?
A-5: Most cases follow autosomal-dominant inheritance with variable age of onset—from childhood to mid-adulthood—and differing treatment intensity within the same family. Genetic confirmation clarifies recurrence risk (each child has a ~50% chance of inheriting the variant) and guides screening: earlier glucose checks, attention to post-meal readings, and pregnancy planning with tighter glycemic goals.
Because stress to the beta-cell accumulates over years, small preventive habits (weight-neutral fitness, adequate sleep, consistent meal timing) can meaningfully slow the march from dysglycemia to medication.
Takeaway: INS-MODY is a beta-cell folding problem more than a classic “not enough insulin” problem. Look for antibody-negative hyperglycemia, family clustering, and a high proinsulin signature. Treat by lowering post-meal stress on the beta-cell and choosing therapies that fit the person’s remaining secretory capacity—then extend that plan to relatives through smart, early screening.
Takeaway: Understanding the Role of INS Mutations in MODY
Mutations in the INS gene are a key contributor to MODY diabetes, impairing insulin production and beta-cell functionality.
These mutations disrupt the folding and secretion of insulin, leading to persistent hyperglycemia.
Accurate genetic testing is essential for early identification, as it helps distinguish MODY from other forms of diabetes and guides treatment decisions.
Targeted strategies, such as the use of sulfonylureas, can effectively manage blood glucose levels without requiring insulin therapy.
Research, such as studies published in Diabetologia, highlights how understanding INS mutations drives advancements in precision medicine, improving outcomes for MODY patients.
References: