How HNF1A Mutations Impair Insulin Secretion?
- admin
- December 5, 2024
- 6:41 pm
- No Comments
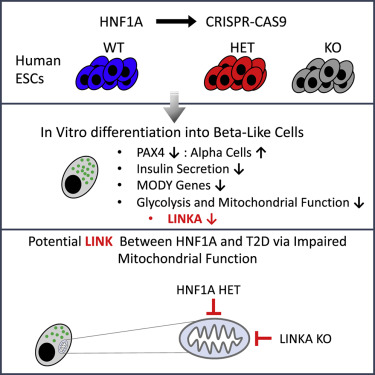
Hepatocyte nuclear factor 1-alpha (HNF1A) is a transcription factor that plays a critical role in pancreatic beta-cell function, which is essential for insulin production and secretion.
Mutations in the HNF1A gene can cause a specific form of monogenic diabetes known as maturity-onset diabetes of the young (MODY), specifically HNF1A-MODY or MODY3.
This article examines how HNF1A mutations impair insulin secretion, exploring the genetic, molecular, and physiological mechanisms behind this condition.
We also address its clinical manifestations and diagnostic considerations to provide a comprehensive understanding of this unique form of diabetes.
Table of Contents:
- Introduction to HNF1A and Its Role in Insulin Secretion
- Genetic Basis of HNF1A Mutations
- Molecular Mechanisms Impairing Insulin Secretion
- 3.1. Disruption of Beta-Cell Development
- 3.2. Impaired Glucose Sensing and Metabolism
- 3.3. Altered Gene Expression in Beta Cells
- Physiological Consequences of HNF1A Mutations
- 4.1. Progressive Beta-Cell Dysfunction
- 4.2. Impact on Glucose Homeostasis
- Clinical Manifestations and Diagnosis
- 5.1. Early-Onset Diabetes Symptoms
- 5.2. Diagnostic Criteria and Genetic Testing
- Conclusion
Introduction to HNF1A and Its Role in Insulin Secretion
HNF1A plays a central role in regulating a wide array of genes essential for the proper development and function of pancreatic beta cells.
These specialized cells are responsible for sensing fluctuations in blood glucose levels and secreting insulin to restore balance.
By activating critical pathways involved in glucose metabolism, insulin synthesis, and secretion, HNF1A ensures beta cells respond effectively to metabolic demands, maintaining glucose homeostasis under normal conditions.
However, mutations in the HNF1A gene disrupt this precise regulatory network.
These mutations can impair the ability of beta cells to sense glucose levels, produce adequate insulin, or release it appropriately, resulting in insufficient insulin secretion.
This dysfunction leads to persistent hyperglycemia, the hallmark of diabetes.
Understanding the mechanisms through which HNF1A mutations affect beta-cell function is vital for developing targeted therapies to improve outcomes for individuals with HNF1A-MODY, a specific form of monogenic diabetes.
Genetic Basis of HNF1A Mutations
HNF1A mutations follow an autosomal dominant inheritance pattern, where the presence of just one mutated copy of the gene is sufficient to cause the condition. These mutations can lead to the production of a defective HNF1A protein or a significant reduction in the gene’s overall expression.
This disruption affects the transcriptional regulation of multiple critical target genes in pancreatic beta cells, impairing their ability to maintain normal insulin secretion.
One major consequence of these mutations is the alteration of HNF1A’s DNA-binding capacity. This impairment prevents the transcription factor from effectively activating genes responsible for key processes like glucose sensing and insulin secretion.
Without proper gene activation, beta cells struggle to function, leading to insufficient insulin production. This deficiency manifests as HNF1A-MODY, characterized by early-onset diabetes and a progressive decline in beta-cell performance over time.
These genetic defects underline the importance of early diagnosis and targeted management strategies.
Molecular Mechanisms Impairing Insulin Secretion
Well, here is how this goes down:
Disruption of Beta-Cell Development:
HNF1A is essential for the development and maturation of pancreatic beta cells. Mutations in HNF1A disrupt normal developmental processes, leading to a reduced number of functional beta cells capable of producing insulin.
This reduction in beta-cell mass directly compromises the insulin reservoir, limiting the body’s ability to regulate blood glucose levels effectively.
Example: A study published in Diabetologia (Thanabalasingham et al., 2022) found that individuals with HNF1A mutations had smaller islet cell clusters and reduced insulin production compared to healthy controls.
This highlights the developmental consequences of HNF1A mutations.
Impaired Glucose Sensing and Metabolism:
Beta cells rely on glucose sensing to regulate insulin secretion. HNF1A mutations impair the expression of genes responsible for glucose metabolism, such as glucose transporters (GLUT2) and key glycolytic enzymes.
This impairment reduces the beta cells’ ability to detect glucose levels accurately, resulting in insufficient insulin release in response to hyperglycemia.
Example: A clinical case reported in Hormone Research in Paediatrics (Galler et al., 2020) demonstrated that individuals with HNF1A mutations exhibited significantly delayed insulin responses after glucose stimulation, supporting the role of HNF1A in glucose sensing.
Altered Gene Expression in Beta Cells:
HNF1A regulates the expression of multiple genes crucial for insulin secretion.
Mutations in HNF1A disrupt the transcriptional control of these genes, leading to defective insulin synthesis and exocytosis.
For instance, reduced expression of the insulin gene itself has been observed in patients with HNF1A mutations.
Example: Research in the International Journal of Molecular Sciences (Miyachi et al., 2022) revealed that HNF1A mutations lead to downregulation of PDX1, a key transcription factor for insulin production. This creates a cascade of reduced insulin gene expression and secretion.
Physiological Consequences of HNF1A Mutations
Let us walk you through this process in brief:
Progressive Beta-Cell Dysfunction:
HNF1A mutations cause a gradual decline in beta-cell function over time. This progressive dysfunction means that individuals with HNF1A-MODY may initially produce sufficient insulin but eventually experience worsening hyperglycemia as their beta cells lose the ability to compensate for glucose elevations.
Example: In a long-term cohort study, individuals with HNF1A-MODY showed a steady decline in their insulin secretion capacity over a 10-year follow-up period, correlating with rising HbA1c levels (Thanabalasingham et al., 2022).
Impact on Glucose Homeostasis:
Impaired insulin secretion disrupts glucose homeostasis, leading to chronic hyperglycemia. This condition increases the risk of diabetes-related complications, such as retinopathy, nephropathy, and cardiovascular disease, particularly in cases where the diabetes is not well-controlled.
Example: A study by Saponaro et al. (2020) noted that patients with HNF1A mutations had higher fasting glucose levels compared to non-MODY diabetic patients, indicating impaired basal insulin secretion.
Clinical Manifestations and Diagnosis
Here is how it all takes shape:
Early-Onset Diabetes Symptoms:
HNF1A-MODY often presents in adolescence or early adulthood with symptoms such as polyuria, polydipsia, and unintended weight loss.
Unlike autoimmune type 1 diabetes, these patients usually retain some beta-cell function, which can delay the onset of severe symptoms.
Example: A 16-year-old patient diagnosed with HNF1A-MODY was initially misdiagnosed with type 2 diabetes due to her mild symptoms and family history. Genetic testing later confirmed the presence of an HNF1A mutation.
Diagnostic Criteria and Genetic Testing:
The diagnosis of HNF1A-MODY requires a combination of clinical assessment and genetic testing.
Key diagnostic features include a family history of early-onset diabetes, non-insulin dependence, and specific biomarkers, such as low C-peptide levels.
Genetic testing identifies mutations in the HNF1A gene, confirming the diagnosis.
Example: Genetic sequencing in a family with multiple cases of early-onset diabetes revealed a shared HNF1A mutation, highlighting the importance of genetic testing for accurate diagnosis and family risk assessment (Galler et al., 2020).
Conclusion
HNF1A mutations impair insulin secretion through several interconnected mechanisms, including disrupted beta-cell development, impaired glucose sensing, and altered gene expression.
These molecular and physiological impairments lead to progressive beta-cell dysfunction and chronic hyperglycemia, characteristic of HNF1A-MODY.
Understanding these pathways is crucial for clinicians and researchers to develop targeted therapies and improve management strategies for individuals with HNF1A-MODY.
Further research is needed to explore potential interventions that can restore beta-cell function and mitigate the long-term complications of this monogenic diabetes subtype.
References: