How Delayed Pancreatic Development Leads to Neonatal Diabetes?
- admin
- December 17, 2024
- 10:19 am
- No Comments
Neonatal diabetes mellitus (NDM) is a rare condition that affects infants within the first six months of life.
Unlike other forms of diabetes, such as Type 1 and Type 2, NDM often arises due to genetic abnormalities or developmental defects in the pancreas.
One such key factor is delayed pancreatic development, which impairs the production and secretion of insulin, leading to persistent hyperglycemia.
This article by bestdietarysupplementfordiabetics.com explores the cellular, genetic, and molecular mechanisms that cause delays in pancreatic organogenesis and beta-cell maturation.
By analyzing the role of transcription factors, signaling pathways, and clinical case studies, we will uncover how these delays culminate in neonatal diabetes.
Pancreatic Development: A Cellular Overview
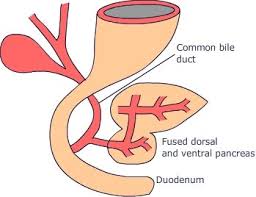
The pancreas begins its development during early embryogenesis, particularly between the fourth and fifth weeks of gestation.
It arises from the foregut endoderm, a tissue layer that eventually gives rise to the pancreas’s dorsal and ventral buds.
As these buds grow, they fuse to form a fully developed pancreas, which houses both exocrine and endocrine components.
The beta cells within the islets of Langerhans are the endocrine units responsible for insulin production.
These cells arise from pancreatic progenitor cells, which differentiate under the influence of critical transcription factors.
The development of the pancreas is a finely tuned process, where even minor delays during the fifth to eighth weeks of gestation can disrupt beta-cell proliferation and maturation.
Disruptions in this critical window of development impair the formation of insulin-secreting cells, resulting in reduced insulin output and neonatal diabetes.
Research in pancreatic organogenesis, such as the work of Pan and Wright (2011), highlights the importance of maintaining proper cellular signaling and gene expression during this phase.
The Role of Genetic Mutations in Delayed Pancreatic Development
Let us walk you through this in brief:
The PDX1 Transcription Factor:
The PDX1 gene, also known as Pancreatic and Duodenal Homeobox 1, is essential for pancreatic development and beta-cell differentiation.
It regulates the expression of insulin and other genes critical to pancreatic function. Mutations in PDX1 cause underdeveloped pancreatic tissue, known as pancreatic hypoplasia, and impair the maturation of beta cells.
In neonates, a mutation in PDX1 leads to a reduction in beta-cell mass, severely limiting insulin production.
Edghill et al. (2008), in their study published in Human Molecular Genetics, revealed that infants with PDX1 mutations develop neonatal diabetes due to the complete or partial loss of pancreatic function.
HNF1B Gene and Organogenesis:
The HNF1B gene, which codes for hepatocyte nuclear factor 1-beta, regulates the development of multiple organs, including the pancreas, kidneys, and liver.
Mutations in HNF1B can lead to Maturity-Onset Diabetes of the Young Type 5 (MODY5) and are associated with pancreatic hypoplasia.
Infants with HNF1B mutations often exhibit a range of developmental defects, including cystic kidney disease and abnormal pancreatic growth.
This genetic abnormality disrupts the proliferation of pancreatic progenitor cells, further impairing beta-cell formation.
In severe cases, the pancreas may fail to develop altogether, causing lifelong insulin dependency.
GATA6 Mutations in Syndromic Cases:
GATA6 is a zinc-finger transcription factor essential for endodermal development, particularly the pancreas and heart.
Mutations in the GATA6 gene are often associated with syndromic neonatal diabetes, where pancreatic hypoplasia is accompanied by additional organ malformations, such as congenital heart disease.
Bonnefond et al. (2012) demonstrated in Nature Genetics that GATA6 mutations disrupt cellular differentiation, preventing the proper formation of beta cells.
Infants with GATA6 mutations often present with severe insulin deficiency and require early medical intervention.
Molecular Signaling Pathways and Pancreatic Delay
Here are the main inputs on this topic:
Disrupted Notch and Wnt Signaling Pathways:
The Notch and Wnt signaling pathways play central roles in the differentiation of pancreatic progenitor cells into endocrine and exocrine lineages.
In a normally developing pancreas, Notch signaling ensures that progenitor cells differentiate into functional beta cells at appropriate stages.
However, disruptions in this signaling process can delay or halt pancreatic development.
Similarly, the Wnt signaling pathway regulates cell proliferation and survival during pancreatic organogenesis.
Mutations or dysregulation in Wnt signaling can lead to a significant reduction in beta-cell mass, resulting in insufficient insulin production.
Studies in Development by Shih et al. (2013) confirm that defective Wnt signaling impairs endocrine differentiation, ultimately contributing to neonatal diabetes.
Defective Hedgehog Pathway Regulation:
The Hedgehog signaling pathway regulates tissue patterning and cell differentiation during pancreatic development.
Overactivation of this pathway suppresses beta-cell progenitor formation, leading to pancreatic hypoplasia.
Research conducted by Hebrok (2003) on mouse models demonstrated that disruptions in Hedgehog signaling delay organogenesis, impairing the pancreas’s ability to produce sufficient insulin.
These findings emphasize the complex molecular interactions required for normal pancreatic growth and how dysregulation contributes to neonatal diabetes.
Impact of Beta-Cell Mass and Function on Neonatal Diabetes
Here is what all you need to know:
Beta-Cell Differentiation and Apoptosis:
Beta cells are the key insulin-secreting cells in the pancreas, and their differentiation is critical for glucose homeostasis.
Delayed pancreatic development often results in a reduced beta-cell mass, limiting the neonate’s ability to produce insulin.
In addition to delayed differentiation, cellular stress caused by genetic mutations or disrupted signaling can induce apoptosis, or programmed cell death, further reducing beta-cell numbers.
Studies highlight that beta cells under prolonged stress—such as that induced by GATA6 mutations—are more susceptible to apoptosis, exacerbating insulin deficiency.
Impact on Insulin Synthesis and Secretion:
Insulin synthesis begins with the transcription of the INS gene, which codes for the proinsulin protein.
Beta cells process proinsulin into mature insulin and C-peptide before secretion. In cases of delayed pancreatic development, impaired beta-cell function disrupts this process, reducing the production and secretion of insulin.
The inability to produce adequate insulin leads to persistent hyperglycemia, a hallmark of neonatal diabetes.
Without intervention, glucose cannot be absorbed efficiently by the body’s cells, leading to failure to thrive and severe metabolic disturbances.
Rare Clinical Case Studies
A quick look at these prospects in detail:
Case of PDX1 Mutation Leading to Severe Pancreatic Hypoplasia:
Emma, a 4-month-old infant, presented with persistent hyperglycemia and severe failure to thrive.
Genetic testing revealed a homozygous mutation in the PDX1 gene, resulting in pancreatic hypoplasia.
Imaging studies confirmed that her pancreas was significantly underdeveloped, and her beta-cell mass was insufficient to produce insulin.
Early intervention with insulin therapy allowed Emma’s glucose levels to stabilize, highlighting the importance of timely diagnosis and treatment in such cases.
Syndromic Neonatal Diabetes in an HNF1B Mutant Infant:
Tom, a 6-month-old infant, exhibited symptoms of diabetes alongside kidney cysts and liver dysfunction.
Genetic testing identified a mutation in the HNF1B gene, linking his pancreatic hypoplasia to MODY5.
Delayed pancreatic development caused significant beta-cell dysfunction, leading to insulin dependence from an early age.
This case underscores the importance of genetic screening in infants presenting with syndromic features, as early diagnosis allows for better clinical management.
FAQs on Delayed Pancreatic Development Leading to Neonatal Diabetes
Q-1: What does “delayed pancreatic development” mean, and how can it cause neonatal diabetes?
A-1: It refers to underformed or immature pancreatic tissue at birth—sometimes a very small gland (hypoplasia) or near-absence (agenesis). With too few or immature β-cells, the newborn can’t release enough insulin, so high blood sugar appears within the first six months of life. Because the exocrine pancreas may also be underdeveloped, some infants show fat malabsorption and poor weight gain alongside hyperglycemia.
Q-2: Which clinical clues suggest underdeveloped pancreas rather than autoimmune diabetes?
A-2: Red flags include hyperglycemia before 6 months, low birth weight or intrauterine growth restriction, negative islet autoantibodies, and signs of malabsorption (greasy stools, poor growth). C-peptide is often present but inappropriately low for glucose levels. Imaging may reveal a very small or absent pancreas. Together, these point toward a developmental cause rather than an autoimmune one.
Q-3: What genes are most often linked to pancreatic hypoplasia/agenesis with neonatal diabetes?
A-3: Variants in transcription-factor genes that orchestrate pancreatic formation are key. Changes in GATA6 are a common cause of pancreatic agenesis. PDX1 and PTF1A variants classically present with neonatal diabetes plus exocrine insufficiency. Broader testing panels also examine genes like RFX6, HNF1B, GCK, KCNJ11, ABCC8, and EIF2AK3 to cover the spectrum of developmental and channel-related etiologies.
Q-4: How is “delayed development” confirmed and distinguished from other neonatal diabetes forms?
A-4: The work-up is stepwise: document early-onset hyperglycemia; check islet autoantibodies; assess exocrine function (e.g., fecal elastase); and image the pancreas by ultrasound or MRI. Genetic testing then narrows the cause. Finding, for example, PTF1A or GATA6 variants together with a small or absent pancreas distinguishes a developmental origin from disorders like KATP-channel mutations.
Q-5: What does management involve when pancreatic development is delayed?
A-5: Treat both endocrine and exocrine sides. Insulin therapy addresses hyperglycemia; pancreatic enzyme replacement with fat-soluble vitamins manages malabsorption when present. Early nutrition support, growth monitoring, and coordinated endocrine–gastroenterology follow-up improve outcomes. Education for caregivers on insulin dosing, hypoglycemia prevention, and enzyme timing is essential.
Q-6: Why are low birth weight and growth issues common, and what are the long-term implications?
A-6: Fetal insulin is a growth factor; if β-cell mass is reduced in utero, the baby often arrives small for gestational age. That early growth constraint can raise later metabolic vulnerability, so careful nutrition, enzyme optimization (if needed), and age-appropriate insulin management matter. With timely diagnosis and dual-track treatment, many infants achieve catch-up growth and stable glycemic control.
Conclusion
Delayed pancreatic development is a significant cause of neonatal diabetes, primarily through impaired beta-cell differentiation, reduced insulin synthesis, and pancreatic hypoplasia.
Genetic mutations in key regulatory genes, such as PDX1, HNF1B, and GATA6, disrupt critical signaling pathways, including Notch, Wnt, and Hedgehog.
These disruptions impair the development and function of insulin-producing beta cells, leading to persistent hyperglycemia in affected infants.
Scientific research and clinical case studies emphasize the importance of early genetic testing to diagnose and manage neonatal diabetes effectively.
While delayed pancreatic development poses challenges, advancements in genetic understanding and targeted therapies continue to improve outcomes for affected neonates.
References: