How Impaired Glucose Metabolism Triggers Neonatal Diabetes?
- admin
- December 20, 2024
- 6:16 pm
- No Comments
Neonatal diabetes is a rare condition that typically presents within the first six months of life and results from genetic or metabolic abnormalities that impair insulin secretion.
One of the primary drivers of this condition is impaired glucose metabolism, which disrupts the body’s ability to regulate blood sugar levels.
In this article, BestDietarySupplementforDiabetics will explore how and why impaired glucose metabolism triggers neonatal diabetes, examining the molecular pathways, genetic influences, and clinical consequences.
We will also incorporate real-life examples to illustrate the profound effects of these metabolic dysfunctions.
Table of Contents:
- Introduction to Neonatal Diabetes and Glucose Metabolism
- The Role of Glucose Metabolism in Insulin Secretion
- Genetic Factors Affecting Glucose Metabolism
- How Impaired Glucose Sensing Leads to Neonatal Diabetes
- Impact on Beta-Cell Function and Insulin Secretion
- Real-Life Case Studies
- Conclusion
Introduction to Neonatal Diabetes and Glucose Metabolism
Neonatal diabetes is characterized by chronic hyperglycemia due to insufficient insulin production.
Unlike other forms of diabetes, it often results from ins gene mutations or defects in metabolic pathways essential for glucose homeostasis.
Understanding the intricate relationship between glucose metabolism and insulin secretion is crucial to diagnosing and managing this rare condition.
The Role of Glucose Metabolism in Insulin Secretion
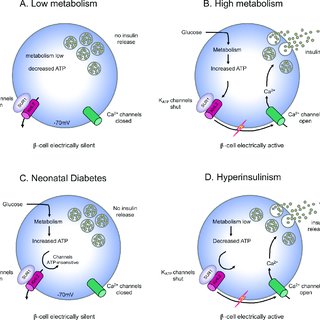
Glucose metabolism is fundamental to the process of insulin secretion, serving as the key trigger for pancreatic beta-cell activity.
When blood glucose levels rise, beta cells metabolize glucose through glycolysis and mitochondrial pathways to generate ATP.
This increased ATP/ADP ratio closes ATP-sensitive potassium (KATP) channels in the beta-cell membrane, causing membrane depolarization.
Depolarization activates voltage-dependent calcium channels, allowing calcium ions to enter the cell. The influx of calcium triggers the exocytosis of insulin granules, ensuring glucose regulation.
Impaired Glucose Metabolism and Insulin Secretion:
Disruptions in glucose metabolism—due to genetic mutations or cellular dysfunction—interrupt this finely tuned process.
Defects in glycolysis, mitochondrial function, or KATP channel activity impair ATP generation and its signaling cascade, leading to reduced insulin release and persistent hyperglycemia.
Scientific Evidence:
Research published by Ashcroft and Rorsman (2004) in Diabetes emphasized the importance of KATP channels in connecting glucose metabolism with insulin secretion.
Their findings revealed how genetic mutations in critical genes, such as KCNJ11 (Kir6.2) and ABCC8 (SUR1), disrupt these mechanisms, causing severe metabolic dysregulation.
Understanding these pathways highlights the complexity of glucose-stimulated insulin secretion and its vulnerability to genetic and metabolic disruptions, particularly in neonatal diabetes.
Genetic Factors Affecting Glucose Metabolism
Genetic mutations are a major underlying cause of impaired glucose metabolism, often leading to neonatal diabetes.
These mutations disrupt critical pathways essential for glucose sensing and insulin secretion.
Mutations in the GCK Gene:
The GCK gene encodes glucokinase, a key enzyme that acts as a glucose sensor in beta cells. Mutations in this gene impair glucose metabolism, reducing ATP production.
Without sufficient ATP, the KATP channel cannot close, and insulin secretion is halted.
Research in Nature Genetics (Hattersley et al., 2018) underscores how GCK mutations contribute to mild and severe forms of neonatal diabetes.
Defects in KCNJ11 and ABCC8:
The KCNJ11 and ABCC8 genes encode Kir6.2 and SUR1, respectively—subunits of the KATP channel.
Mutations in these genes interfere with the channel’s ability to respond to ATP levels, causing either persistent channel opening or closure.
This leads to disrupted insulin secretion and glucose imbalance.
INS Gene Mutations:
The INS gene encodes insulin. Mutations result in misfolded insulin precursors, triggering beta-cell stress and reducing insulin output.
Research published in Human Molecular Genetics (Edghill et al., 2008) highlighted the critical impact of these mutations on neonatal glucose regulation.
Together, these genetic abnormalities disrupt the intricate mechanisms of glucose metabolism, underscoring the role of genetic testing in early diagnosis and intervention.
Example:
A study published in Nature Genetics (Gloyn et al., 2004) identified mutations in KCNJ11 and ABCC8 as primary causes of neonatal diabetes, emphasizing the genetic basis of impaired glucose metabolism.
How Impaired Glucose Sensing Leads to Neonatal Diabetes?
Impaired glucose sensing plays a critical role in the development of neonatal diabetes by disrupting the tightly regulated mechanism of insulin secretion.
Disruption of ATP Generation:
In normal beta-cell function, glucose enters the cell and undergoes glycolysis, generating ATP. This process triggers the closure of KATP channels, leading to membrane depolarization, calcium influx, and subsequent insulin release.
However, in neonates with impaired glucose metabolism, defects in glycolysis or mitochondrial function significantly reduce ATP production. This failure to generate adequate ATP halts the signaling cascade needed for insulin secretion.
A study published in Endocrine Reviews (Henquin, 2000) emphasized how insufficient ATP production impairs the beta cell’s ability to respond to glucose, effectively disrupting insulin output.
Persistent Hyperglycemia:
The lack of insulin leads to persistent hyperglycemia, as glucose cannot enter cells for energy.
Elevated blood glucose levels exacerbate metabolic imbalances and place significant stress on neonatal physiology.
Persistent hyperglycemia is linked to failure to thrive, developmental delays, and an increased risk of long-term complications, including organ damage.
Together, these mechanisms illustrate how impaired glucose sensing results in neonatal diabetes, highlighting the essential role of beta-cell ATP production in maintaining glucose homeostasis.
Impact on Beta-Cell Function and Insulin Secretion
Impaired glucose metabolism significantly damages beta cells through two primary mechanisms, both of which disrupt their ability to secrete insulin effectively.
Cellular Stress and Apoptosis:
Chronic hyperglycemia exposes beta cells to oxidative stress, a condition where excessive reactive oxygen species (ROS) accumulate.
This oxidative burden damages cellular components, including DNA, proteins, and lipids, leading to beta-cell apoptosis (programmed cell death).
A pivotal study published in Diabetes (Rutter et al., 2010) demonstrated that oxidative stress resulting from impaired glucose metabolism drastically reduces beta-cell viability.
Over time, the loss of beta cells exacerbates insulin insufficiency, creating a vicious cycle of hyperglycemia and metabolic dysfunction.
Endoplasmic Reticulum (ER) Stress:
Beta cells rely on the endoplasmic reticulum to fold and process insulin properly.
When glucose metabolism is impaired, defective insulin molecules accumulate in the ER, triggering stress and the unfolded protein response (UPR). Prolonged ER stress overwhelms the UPR, leading to beta-cell dysfunction and apoptosis.
A study in Human Molecular Genetics (Edghill et al., 2008) focused on mutations in the INS gene, highlighting how misfolded insulin proteins amplify ER stress and compromise beta-cell function.
Together, these mechanisms reveal how impaired glucose metabolism causes progressive beta-cell failure, underscoring its critical role in neonatal diabetes development.
Real-Life Case Studies
Case Study 1: Neonatal Diabetes Due to GCK Mutation:
Emma, a three-month-old infant, presented with severe hyperglycemia. Genetic testing revealed a mutation in the GCK gene, impairing glucose sensing and ATP production.
Early diagnosis allowed her to transition from insulin injections to a tailored treatment plan, which stabilized her glucose levels and improved her quality of life.
Case Study 2: Familial Neonatal Diabetes Linked to KCNJ11 Mutation:
Liam, a five-month-old baby, was diagnosed with neonatal diabetes after persistent hyperglycemia. Genetic analysis identified a KCNJ11 mutation that kept KATP channels open, blocking insulin secretion.
Sulfonylurea therapy successfully restored partial insulin secretion. Interestingly, Liam’s father carried the same mutation but developed diabetes later in life, illustrating the genetic and phenotypic variability of the condition.
Case Study 3: INS Gene Mutation and Beta-Cell Failure:
Sophia, a six-month-old diagnosed with neonatal diabetes, had a mutation in the INS gene.
This mutation caused the accumulation of misfolded insulin in the ER, leading to beta-cell apoptosis.
Insulin therapy was initiated, but her condition highlighted the challenges of managing severe genetic beta-cell dysfunction.
Conclusion
Impaired glucose metabolism lies at the core of neonatal diabetes, disrupting the tightly regulated processes that govern insulin secretion.
Genetic mutations in key regulatory pathways further exacerbate this dysfunction, leading to persistent hyperglycemia and significant health challenges.
By understanding these mechanisms and leveraging advances in genetic testing, healthcare providers can offer targeted treatments that improve outcomes for neonates with diabetes.
References: