How ABCC8 Mutations Disrupt KATP Channel Function?
- admin
- December 26, 2024
- 4:09 pm
- No Comments
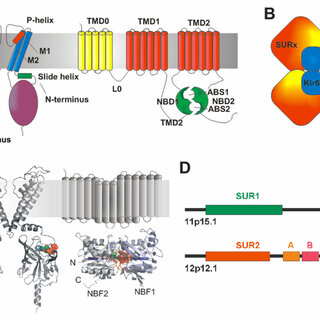
ATP-sensitive potassium (KATP) channels are crucial in regulating cellular energy balance and insulin secretion, particularly in pancreatic beta cells.
These channels consist of two primary subunits: Kir6.2, encoded by the KCNJ11 gene, and SUR1, encoded by the ABCC8 gene.
Mutations in the ABCC8 gene impair the function of the SUR1 subunit, leading to significant disruptions in KATP channel function.
This article explores the molecular mechanisms by which ABCC8 mutations affect KATP channel activity, the physiological consequences, and real-life clinical presentations.
Article Index:
- Introduction to KATP Channels and Their Role in Cellular Physiology
- Structure and Function of the SUR1 Subunit Encoded by ABCC8
- Mechanisms of ABCC8 Mutations on KATP Channel Function
- Types of ABCC8 Mutations and Their Specific Impacts
- Physiological Consequences of Disrupted KATP Channel Function
- Clinical Manifestations of ABCC8 Mutations
- Scientific Evidence Supporting the Impact of ABCC8 Mutations
- Real-Life Examples: Case Studies
- Conclusion: The Broad Impact of ABCC8 Mutations on Cellular and Systemic Function
Introduction to KATP Channels and Their Role in Cellular Physiology
KATP channels are essential molecular sensors that link cellular metabolism to electrical activity.
Located in pancreatic beta cells, cardiac muscle, and neurons, these channels regulate insulin secretion, heart rate, and neuronal excitability.
They function by allowing potassium ions to flow out of the cell, maintaining a hyperpolarized membrane potential under low ATP conditions.
When ATP levels rise, the channel closes, leading to membrane depolarization and triggering downstream physiological processes such as insulin release.
Mutations in the ABCC8 gene disrupt the SUR1 subunit, impairing the channel’s ability to respond to ATP and ADP.
These disruptions lead to a range of conditions, from hyperinsulinism to diabetes, depending on whether the mutation results in a gain or loss of function.
Structure and Function of the SUR1 Subunit Encoded by ABCC8
The SUR1 subunit plays a regulatory role in the KATP channel by sensing metabolic signals and modulating the channel’s activity accordingly.
SUR1, a member of the ATP-binding cassette (ABC) transporter family, contains two nucleotide-binding domains (NBDs) and two transmembrane domains (TMDs).
The NBDs bind and hydrolyze ATP, which helps regulate the opening and closing of the KATP channel. SUR1 also interacts with sulfonylureas, drugs used to treat diabetes by promoting insulin secretion.
Mutations in the ABCC8 gene can alter the structure or function of SUR1, impairing its ability to respond to ATP and ADP, which disrupts normal KATP channel function.
These alterations are central to the development of diseases such as congenital hyperinsulinism and neonatal diabetes.
Mechanisms of ABCC8 Mutations on KATP Channel Function
Mutations in the ABCC8 gene can disrupt the function of ATP-sensitive potassium (KATP) channels through several molecular mechanisms.
These disruptions impact the SUR1 subunit’s ability to regulate the channel appropriately, leading to significant physiological consequences.
- Reduced Sensitivity to ATP: Certain mutations impair SUR1’s capacity to sense intracellular ATP levels effectively. This defect prevents the channel from closing in response to rising ATP concentrations, resulting in persistent potassium efflux. This prolonged open state inhibits the membrane depolarization required for insulin secretion, contributing to hyperglycemia and diabetes.
- Impaired Interaction with ADP: Some mutations hinder the ability of SUR1 to respond to ADP, which is crucial for channel regulation during metabolic stress. This loss of responsiveness disrupts the balance between open and closed states of the channel, further impairing its functionality.
- Defective Drug Binding: Mutations can also affect SUR1’s ability to bind sulfonylureas, drugs designed to close KATP channels and stimulate insulin secretion. This defect reduces the efficacy of these medications, complicating treatment options for individuals with related conditions.
These molecular changes profoundly influence the KATP channel’s ability to regulate insulin secretion, leading to a spectrum of disorders ranging from congenital hyperinsulinism to neonatal diabetes.
Understanding these mechanisms is critical for developing targeted therapies.
Types of ABCC8 Mutations and Their Specific Impacts
Mutations in ABCC8 can be broadly classified into gain-of-function and loss-of-function mutations, each with distinct physiological consequences:
- Gain-of-Function Mutations:
- Mutations such as E1506K increase the likelihood of the channel remaining open, causing insufficient insulin secretion and neonatal diabetes.
- These mutations impair the channel’s ability to close in response to ATP, leading to chronic hyperglycemia.
- Loss-of-Function Mutations:
- Mutations like V187D reduce the channel’s ability to open in response to low ATP, causing excessive insulin secretion and congenital hyperinsulinism.
- These mutations make the channel overly sensitive to ATP, preventing normal potassium efflux and maintaining a depolarized membrane state.
Physiological Consequences of Disrupted KATP Channel Function
The physiological impacts of ABCC8 mutations are most evident in pancreatic beta cells but also extend to other tissues where KATP channels are crucial for maintaining cellular function and metabolic balance:
- In Pancreatic Beta Cells: The disruption of KATP channel function caused by ABCC8 mutations directly affects insulin secretion. Depending on the mutation’s nature, it can lead to either excessive insulin release, resulting in hypoglycemia (as seen in congenital hyperinsulinism), or insufficient secretion, leading to hyperglycemia (as in neonatal diabetes).
- In the Heart: KATP channels in cardiac cells help the heart adapt to metabolic stress by regulating ion flow during ischemia or oxygen deprivation. Abnormal activity due to ABCC8 mutations can impair this adaptive response, potentially leading to arrhythmias or other cardiac complications.
- In Neurons: Neuronal KATP channels play a role in controlling excitability and protecting against overactivation during metabolic stress. Dysfunction in these channels, as seen with certain ABCC8 mutations, can lead to seizures, developmental delays, and other neurological symptoms.
These widespread physiological effects highlight the essential role of ABCC8 and the KATP channel in various tissues, emphasizing the need for targeted therapeutic approaches.
Clinical Manifestations of ABCC8 Mutations
Mutations in the ABCC8 gene can result in a wide range of clinical conditions, each with distinct symptoms and management challenges:
- Congenital Hyperinsulinism: This condition is marked by excessive insulin secretion, leading to severe hypoglycemia. It often presents in infancy and may result in recurrent episodes of low blood sugar, which can impair brain function if untreated. Management typically involves medication to suppress insulin secretion, but severe cases may require surgical removal of part of the pancreas.
- Neonatal Diabetes: Caused by insufficient insulin secretion, neonatal diabetes manifests as hyperglycemia within the first six months of life. Genetic testing often reveals ABCC8 mutations, and treatment with sulfonylureas can restore KATP channel function, significantly improving glucose regulation in many cases.
- DEND Syndrome: Standing for developmental delay, epilepsy, and neonatal diabetes, this rare and severe condition arises from mutations affecting KATP channels in both pancreatic beta cells and neurons. It combines metabolic dysfunction with neurological symptoms, requiring a multidisciplinary approach for management.
These diverse conditions highlight the critical role of ABCC8 in maintaining glucose homeostasis and neural function, emphasizing the importance of early diagnosis and targeted treatment strategies.
Scientific Evidence Supporting the Impact of ABCC8 Mutations
Research has provided robust evidence linking ABCC8 mutations to disrupted KATP channel function:
- Flanagan et al., 2006: This study in The New England Journal of Medicine identified mutations in ABCC8 as a cause of neonatal diabetes, demonstrating how these mutations impair channel closure.
- Dunne et al., 2004: Published in Diabetologia, this research highlighted the role of ABCC8 mutations in congenital hyperinsulinism and their effect on insulin secretion.
- Pearson et al., 2006: This study in Diabetes explored the therapeutic potential of sulfonylureas in treating ABCC8-related neonatal diabetes, emphasizing the functional impact of these mutations.
Two Real Life Examples on This Topic:
A quick look at each of these in brief:
Example 1: Neonatal Diabetes in Infancy
Emma, a two-month-old infant, was admitted to the hospital with severe hyperglycemia and failure to thrive.
Her parents were distressed as doctors ran a battery of tests to determine the cause. Genetic testing identified an E1506K mutation in the ABCC8 gene, which confirmed a diagnosis of neonatal diabetes.
This mutation disrupted the KATP channel function, preventing adequate insulin secretion. Emma’s treatment team transitioned her from insulin injections to sulfonylureas, a class of drugs known to restore KATP channel function by promoting channel closure.
This approach successfully normalized her glucose levels, enabling her to grow and thrive like any healthy infant.
Example 2: Congenital Hyperinsulinism
James, a one-year-old boy, experienced recurrent episodes of severe hypoglycemia that often left him lethargic and irritable. After repeated hospital visits and extensive evaluations, genetic analysis revealed a V187D mutation in the ABCC8 gene.
This mutation caused the KATP channels to remain closed, leading to excessive insulin secretion and dangerously low blood sugar levels. Although medication partially controlled James’ symptoms, his condition necessitated surgical intervention to remove a portion of his pancreas.
Post-surgery, James’ hypoglycemic episodes reduced significantly, but he continues to be closely monitored to ensure stable glucose levels. These cases highlight the critical impact of ABCC8 mutations on KATP channel function and their varying clinical presentations.
The Broad Impact of ABCC8 Mutations on Cellular and Systemic Function
Mutations in the ABCC8 gene profoundly disrupt KATP channel function, impairing the balance between metabolic signals and electrical activity.
These disruptions lead to a range of conditions, from hypoglycemia in congenital hyperinsulinism to hyperglycemia in neonatal diabetes.
Understanding the molecular mechanisms underlying these mutations is critical for developing targeted therapies, such as sulfonylureas, which have shown success in managing some cases.
Continued research will enhance our ability to diagnose and treat these conditions, improving outcomes for affected individuals.
References: