Genetic Mechanisms Underlying Rare Causes of Neonatal Diabetes?
- admin
- December 29, 2024
- 12:19 pm
- No Comments
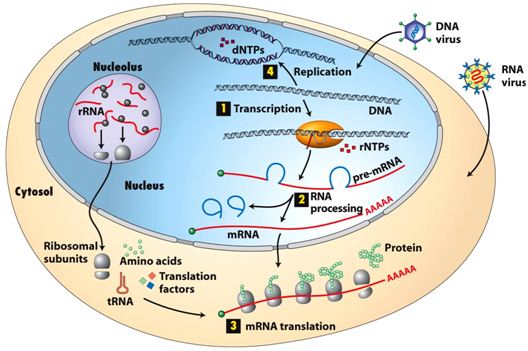
Neonatal diabetes is a rare condition that manifests within the first six months of life, characterized by hyperglycemia due to insufficient insulin secretion.
While the majority of cases are linked to well-known genetic mutations (such as INS gene mutation), some are caused by rare and poorly understood genetic mechanisms.
These rare causes provide valuable insights into the molecular pathways that regulate pancreatic beta-cell development and insulin production.
This article by bestdietarysupplementfordiabetics.com explores the genetic mechanisms underlying rare causes of neonatal diabetes, supported by scientific evidence and real-life examples, to provide a comprehensive understanding of this complex condition.
Article Index:
- Introduction to Neonatal Diabetes
- Overview of Genetic Mechanisms in Neonatal Diabetes
- Rare Monogenic Mutations
- Epigenetic Alterations and Neonatal Diabetes
- Chromosomal Abnormalities Linked to Neonatal Diabetes
- Mitochondrial Mutations and Their Impact
- Role of Rare Transcription Factor Mutations
- Case Studies of Rare Neonatal Diabetes Causes
- Scientific Evidence Supporting Rare Genetic Mechanisms
- FAQs on Genetic Mechanisms in Neonatal Diabetes
- Conclusion: Expanding Our Understanding of Neonatal Diabetes
Introduction to Neonatal Diabetes
Neonatal diabetes is a rare and serious condition, occurring in approximately 1 in 100,000 live births.
Unlike type 1 diabetes, which results from autoimmune destruction of pancreatic beta cells, neonatal diabetes is caused by genetic mutations that impair insulin production or secretion.
This condition typically manifests within the first six months of life, presenting with persistent hyperglycemia.
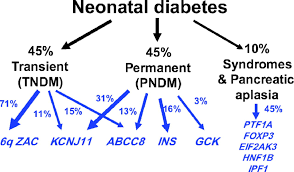
Neonatal diabetes can be classified into two types: transient neonatal diabetes mellitus (TNDM), which resolves during early childhood but may recur later in life, and permanent neonatal diabetes mellitus (PNDM), which requires lifelong management.
Identifying the specific genetic mutations underlying neonatal diabetes is essential not only for accurate diagnosis and effective treatment but also for gaining insights into the broader mechanisms of pancreatic beta-cell dysfunction and glucose regulation.
Early genetic screening and intervention can significantly improve patient outcomes and quality of life.
Overview of Genetic Mechanisms in Neonatal Diabetes
Genetic mutations underlying neonatal diabetes often disrupt critical pathways essential for normal glucose regulation. These mutations primarily affect genes involved in:
- Pancreatic Beta-Cell Development: Genes crucial for the formation and maturation of insulin-producing beta cells can result in inadequate insulin secretion when mutated.
- Insulin Synthesis and Secretion: Mutations in genes responsible for producing or releasing insulin impair the body’s ability to maintain blood glucose levels.
- ATP-Sensitive Potassium (KATP) Channel Function: Mutations in the KCNJ11 and ABCC8 genes, which encode components of the KATP channel, disrupt the cell’s ability to link metabolic signals with insulin release.
While mutations in KCNJ11 and ABCC8 genes account for the majority of neonatal diabetes cases, rare genetic mechanisms provide additional insights into the condition’s complexity.
These less common mutations illuminate diverse molecular pathways, highlighting the intricate processes governing glucose homeostasis and insulin regulation.
Understanding these mechanisms is essential for accurate diagnosis and targeted treatments.
Rare Monogenic Mutations
Rare monogenic mutations are single-gene defects that disrupt essential pathways in insulin production or beta-cell function, leading to neonatal diabetes.
These mutations, though rare, provide critical insights into the molecular mechanisms underlying this condition.
- INS Gene Mutations: Mutations in the INS gene, which encodes the insulin hormone, impair its synthesis or structural integrity, directly causing insulin deficiency. A study by Edghill et al. (2008) in Diabetes identified several INS mutations associated with early-onset diabetes, emphasizing the gene’s importance in beta-cell function.
- GATA6 and GATA4 Mutations: These transcription factors are vital for pancreatic development. Mutations lead to pancreatic hypoplasia, significantly reducing insulin production and contributing to neonatal diabetes.
- EIF2AK3 Mutations: This mutation, linked to Wolcott-Rallison syndrome, affects beta-cell survival under metabolic stress, compounding the challenges of insulin regulation.
Example:
David, a six-month-old infant, was diagnosed with neonatal diabetes caused by a rare INS gene mutation.
Genetic testing facilitated an early diagnosis, allowing timely intervention with insulin therapy to stabilize his glucose levels.
Such cases highlight the importance of genetic screening in diagnosing and managing monogenic diabetes effectively.
Epigenetic Alterations and Neonatal Diabetes
Epigenetic changes, including DNA methylation and histone modifications, play a critical role in regulating gene expression without altering the DNA sequence itself.
In some instances, neonatal diabetes results from disruptions in these regulatory mechanisms, affecting key genes involved in beta-cell function and insulin secretion.
- ZFP57 Mutations: These mutations disrupt imprinting control regions, leading to the abnormal expression of imprinted genes essential for proper beta-cell development and function. Such dysregulation can impair insulin secretion, contributing to neonatal diabetes.
- Hypomethylation Syndromes: Conditions like transient neonatal diabetes mellitus (TNDM) are often linked to hypomethylation at the PLAGL1/HYMAI locus, which alters gene expression patterns necessary for maintaining glucose homeostasis.
Example:
Emma, a newborn diagnosed with TNDM, was found to have hypomethylation at the PLAGL1 locus.
Her condition resolved by age two, illustrating the transient nature of epigenetically driven diabetes.
Studies like those published by Docherty et al. (2010) in Diabetes have emphasized the importance of understanding epigenetic influences for accurate diagnosis and management of neonatal diabetes cases.
Chromosomal Abnormalities Linked to Neonatal Diabetes
Structural chromosomal abnormalities are significant contributors to neonatal diabetes, disrupting essential genetic loci involved in insulin production and beta-cell function.
These abnormalities can lead to conditions such as transient neonatal diabetes mellitus (TNDM), where insulin secretion is impaired during infancy but may resolve later in childhood.
- 6q24 Abnormalities: Duplications or uniparental disomy (both copies of a chromosome inherited from one parent) at the 6q24 locus are among the most common causes of TNDM. These genetic disruptions alter the regulation of imprinted genes critical for beta-cell activity.
- Translocations and Deletions: Chromosomal rearrangements can affect genes involved in pancreatic development, leading to long-term or transient insulin secretion deficiencies.
Example:
A case study published in Pediatrics (2012) described a newborn diagnosed with TNDM caused by a duplication at the 6q24 locus.
The use of chromosomal analysis facilitated an early and accurate diagnosis, enabling tailored treatment that stabilized the infant’s blood glucose levels.
This case underscores the importance of advanced genetic testing in identifying chromosomal abnormalities and guiding effective clinical management.
Mitochondrial Mutations and Their Impact
Mitochondria play a crucial role in ATP production, which regulates insulin secretion. Rare mitochondrial mutations can disrupt this process, leading to diabetes.
- MT-TL1 Mutations: These mutations impair mitochondrial tRNA function, reducing ATP production and insulin release.
- Maternally Inherited Diabetes and Deafness (MIDD): Caused by specific mitochondrial mutations, MIDD can present as neonatal diabetes in severe cases.
Example:
Mark, a newborn with neonatal diabetes and hearing loss, was found to have an MT-TL1 mutation, linking his condition to mitochondrial dysfunction.
Role of Rare Transcription Factor Mutations
Transcription factors play a vital role in regulating genes essential for pancreatic development and insulin secretion.
Rare mutations in these factors can disrupt normal beta-cell formation and function, resulting in neonatal diabetes.
- HNF1B Mutations: These mutations impact both pancreatic and renal development, leading to a dual presentation of diabetes and kidney dysfunction. Individuals with HNF1B mutations often exhibit a spectrum of symptoms, including renal cysts and impaired insulin production.
- RFX6 Mutations: Critical for beta-cell differentiation, RFX6 mutations hinder the maturation of insulin-producing cells, significantly reducing insulin secretion and causing early-onset diabetes.
Example:
Lily, a one-year-old girl, was diagnosed with neonatal diabetes and renal cysts. Genetic testing identified an HNF1B mutation as the underlying cause.
This diagnosis provided clarity, and genetic counseling helped her family understand the hereditary nature of her condition.
Studies like those by Ellard et al. (2013) emphasize the importance of early genetic analysis for rare transcription factor mutations, enabling accurate diagnosis and tailored management strategies for affected individuals.
Case Studies of Rare Neonatal Diabetes Causes
Let us walk you through a few classic examples:
Case Study 1: Genetic Mosaicism
John, a six-month-old infant, presented with mild hyperglycemia that initially puzzled his healthcare providers.
After standard diagnostic approaches failed to pinpoint the cause, genetic testing revealed mosaicism involving the KCNJ11 gene. Mosaicism occurs when a genetic mutation is present in some, but not all, of an individual’s cells, leading to variable clinical presentations.
In John’s case, the mosaic mutation partially impaired the function of the ATP-sensitive potassium (KATP) channels in pancreatic beta cells, resulting in inconsistent insulin secretion.
This rare presentation complicated both diagnosis and treatment, underscoring the importance of comprehensive genetic testing. Studies, such as those by Gloyn et al. (2004), have highlighted mosaicism as an underrecognized factor in neonatal diabetes, emphasizing its diagnostic challenges and implications for personalized care.
Case Study 2: Environmental Modifier Interaction
Sophia, a newborn diagnosed with neonatal diabetes, was found to have a rare mutation in the GCK gene, which encodes glucokinase—a key glucose sensor in beta cells.
Genetic analysis revealed that her condition was exacerbated by prenatal exposure to maternal hyperglycemia during pregnancy.
This interplay between genetics and environment highlighted the significant role environmental modifiers can play in the expression of genetic conditions.
Sophia’s case illustrates the importance of considering both genetic and prenatal environmental factors in diagnosing and managing neonatal diabetes.
Research by Ellard et al. (2013) supports the concept that such interactions can influence the onset and severity of genetic disorders, underscoring the need for holistic approaches to patient care.
Scientific Evidence Supporting Rare Genetic Mechanisms
- Gloyn et al. (2004): Identified novel KCNJ11 mutations in neonatal diabetes, broadening the understanding of monogenic causes.
- Hattersley et al. (2006): Highlighted the diagnostic value of genetic testing in neonatal diabetes management.
- Ellard et al. (2013): Reviewed the spectrum of genetic mutations causing neonatal diabetes, including rare cases involving chromosomal abnormalities and epigenetic changes.
These studies underscore the importance of research in uncovering rare genetic mechanisms.
FAQs on Genetic Mechanisms in Neonatal Diabetes
Q-1: What are the primary genetic mechanisms underlying rare causes of neonatal diabetes?
A-1: Neonatal diabetes, diagnosed before six months of age, is primarily caused by genetic mutations affecting insulin secretion. The most common mechanisms include:
K-ATP Channel Mutations: Alterations in the KCNJ11 and ABCC8 genes, encoding the Kir6.2 and SUR1 subunits of the ATP-sensitive potassium channel, respectively, lead to impaired insulin secretion.
Insulin Gene Mutations: Defects in the INS gene result in misfolded insulin, causing endoplasmic reticulum stress and beta-cell dysfunction.
6q24 Gene Overexpression: Epigenetic changes, such as hypomethylation, lead to overexpression of genes at the 6q24 locus, causing transient neonatal diabetes.
Other Genetic Factors: Mutations in genes like EIF2AK3, FOXP3, and YIPF5 have been associated with neonatal diabetes, often presenting with additional clinical features.
Q-2: How do mutations in the KCNJ11 and ABCC8 genes contribute to neonatal diabetes?
A-2: Mutations in the KCNJ11 and ABCC8 genes disrupt the function of the K-ATP channel in pancreatic beta cells. These channels regulate insulin secretion in response to glucose levels. Mutations can cause the channel to remain open, preventing insulin release and leading to hyperglycemia. Depending on the mutation’s nature, this can result in transient or permanent neonatal diabetes.
Q-3: What role do insulin gene mutations play in neonatal diabetes?
A-3: Mutations in the INS gene lead to the production of misfolded insulin molecules. These misfolded proteins accumulate in the endoplasmic reticulum, causing cellular stress and beta-cell apoptosis. This dysfunction impairs insulin secretion, resulting in hyperglycemia. Such mutations are a significant cause of permanent neonatal diabetes.
Q-4: Can you explain the genetic mechanism behind 6q24-related transient neonatal diabetes?
A-4: 6q24-related transient neonatal diabetes is caused by epigenetic changes, such as hypomethylation, leading to overexpression of genes at the 6q24 locus. This overexpression disrupts normal pancreatic development and function, resulting in transient hyperglycemia. The condition often resolves in early childhood but can relapse later in life.
Q-5: What is the significance of EIF2AK3 and FOXP3 mutations in neonatal diabetes?
A-5: Mutations in the EIF2AK3 gene are associated with Wolfram syndrome, characterized by neonatal diabetes, optic atrophy, and deafness. FOXP3 mutations are linked to immune dysregulation, leading to autoimmune diabetes. Both mutations highlight the complex genetic mechanisms underlying neonatal diabetes and its associated features.
Q-6: How do YIPF5 gene mutations affect neonatal diabetes?
A-6: Mutations in the YIPF5 gene disrupt vesicular trafficking within beta cells, leading to endoplasmic reticulum stress and accumulation of proinsulin. This dysfunction impairs insulin secretion, resulting in neonatal diabetes. Additionally, YIPF5 mutations are associated with microcephaly and epilepsy, indicating a broader impact on cellular processes.
Q-7: Are there other rare genetic causes of neonatal diabetes?
A-7: Yes, other rare genetic causes include mutations in the GCK gene, leading to permanent neonatal diabetes, and in the PDX1 gene, affecting pancreatic development. Additionally, chromosomal abnormalities like the 17q12 microdeletion syndrome can result in neonatal diabetes along with other systemic issues. These diverse genetic mechanisms underscore the complexity of neonatal diabetes etiology.
Conclusion: Expanding Our Understanding of Neonatal Diabetes
The genetic mechanisms underlying rare causes of neonatal diabetes highlight the complexity of this condition.
From monogenic mutations to epigenetic alterations and chromosomal abnormalities, each discovery provides valuable insights into pancreatic beta-cell function and insulin regulation.
Advancements in genetic testing and research have significantly improved diagnostic accuracy and treatment strategies.
Continued exploration of rare genetic mechanisms will further enhance our understanding of neonatal diabetes, paving the way for personalized medicine and better patient outcomes.
References: