How WFS1 Mutations Disrupt Endoplasmic Reticulum (ER) Function in Wolfram Syndrome?
- admin
- January 5, 2025
- 1:28 pm
- No Comments
Wolfram Syndrome is a rare, progressive disorder characterized by diabetes mellitus, optic atrophy, hearing loss, and neurodegeneration.
Central to its pathology are mutations in the WFS1 gene, which encodes the protein wolframin, a critical regulator of endoplasmic reticulum (ER) function.
This article by bestdietarysupplementfordiabetics.com delves into how WFS1 mutations disrupt ER homeostasis, exploring the underlying mechanisms and their physiological consequences.
We will discuss protein misfolding, calcium dysregulation, ER stress, and apoptosis, supported by scientific evidence and real-life examples.
Article Index
- Introduction to WFS1 and Its Role in the ER
- Protein Misfolding and ER Stress
- Calcium Dysregulation in Wolfram Syndrome
- Activation of the Unfolded Protein Response (UPR)
- ER-Associated Apoptosis in WFS1 Mutations
- Systemic Effects of ER Dysfunction
- Case Examples: Real-Life Impacts
- FAQs on WFS1 Mutation and ER Stress in Wolfram Syndrome
- Conclusion
Introduction to WFS1 and Its Role in the ER
The WFS1 gene encodes wolframin, a transmembrane protein located in the ER. Wolframin is integral to maintaining ER homeostasis, particularly in managing protein folding, calcium storage, and cellular stress responses.
Mutations in WFS1 disrupt these processes, triggering widespread cellular dysfunction and progressive tissue damage in Wolfram Syndrome.
A study published in Nature Genetics (Inoue et al., 1998) first linked WFS1 mutations to ER dysfunction, highlighting the critical role of wolframin in cellular health.
Protein Misfolding and ER Stress
A quick look at this aspect in brief:
How Protein Misfolding Occurs?
In the ER, newly synthesized proteins are folded into their functional conformations. WFS1 mutations impair wolframin’s role in assisting protein folding, leading to the accumulation of misfolded proteins.
These misfolded proteins accumulate in the ER lumen, causing ER stress—a condition linked to cellular dysfunction.
Scientific Insight
Research in Molecular and Cellular Biology (Fonseca et al., 2010) demonstrated that cells lacking functional WFS1 exhibited an overload of misfolded proteins, triggering stress pathways. The inability to efficiently process proteins compromises the ER’s capacity to perform its core functions.
Example:
Anna, a 12-year-old with Wolfram Syndrome, experienced progressive vision loss due to optic nerve degeneration. Genetic testing revealed a WFS1 mutation disrupting protein processing in retinal cells, contributing to cellular stress and neurodegeneration.
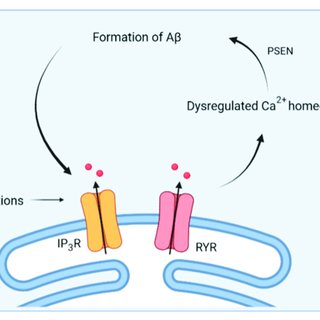
Calcium Dysregulation in Wolfram Syndrome
Let us walk you through the process in brief:
Wolframin’s Role in Calcium Storage:
The ER is a major reservoir of intracellular calcium, essential for signaling pathways, protein folding, and cell survival. Wolframin helps regulate calcium levels within the ER, maintaining cellular homeostasis. WFS1 mutations disrupt this balance, leading to calcium leakage and impaired signaling.
Consequences of Calcium Dysregulation:
Calcium leakage weakens cellular processes, exacerbating ER stress and increasing susceptibility to apoptosis. The dysregulation of calcium signaling particularly affects beta cells in the pancreas, contributing to diabetes in Wolfram Syndrome.
Scientific Evidence:
A study in Diabetes (Zatyka et al., 2011) linked WFS1 mutations to calcium handling defects in pancreatic beta cells, impairing insulin secretion and leading to early-onset diabetes.
Example:
Tom, a 10-year-old boy, was diagnosed with Wolfram Syndrome after developing diabetes and progressive hearing loss. Testing revealed dysfunctional calcium signaling in both pancreatic beta cells and cochlear neurons, highlighting the systemic impact of ER dysfunction.
Activation of the Unfolded Protein Response (UPR)
The unfolded protein response (UPR) is a vital cellular defense mechanism that is activated to restore homeostasis in the endoplasmic reticulum (ER) during periods of stress.
The UPR achieves this by halting protein synthesis, increasing the degradation of misfolded proteins, and enhancing the production of molecular chaperones, which assist in proper protein folding.
However, in Wolfram Syndrome, mutations in the WFS1 gene disrupt this delicate balance. WFS1, a key ER-resident protein, normally helps regulate UPR signaling and calcium homeostasis.
When WFS1 is absent or dysfunctional, the UPR becomes chronically activated, failing to resolve the underlying stress. This prolonged stress overwhelms cellular repair systems, leading to impaired function and eventual apoptosis.
Scientific Evidence:
Research published in Nature Reviews Endocrinology (Fonseca et al., 2013) demonstrated that cells lacking WFS1 show hyperactivation of the UPR, particularly in the IRE1α and PERK pathways, which are major arms of the UPR.
This sustained signaling was linked to apoptotic cell death in vulnerable tissues such as pancreatic beta cells and neurons.
The study highlighted how dysregulated UPR in WFS1-deficient cells contributes to progressive tissue damage, underscoring its role in the pathology of Wolfram Syndrome.
ER-Associated Apoptosis in WFS1 Mutations
When endoplasmic reticulum (ER) stress becomes chronic and unmanageable, the unfolded protein response (UPR) shifts from a protective mechanism to a pro-apoptotic pathway.
Initially, the UPR aims to restore ER homeostasis by halting protein synthesis, degrading misfolded proteins, and increasing the production of molecular chaperones.
However, persistent stress overwhelms this system, leading to the activation of CHOP (C/EBP homologous protein) and caspase cascades, which are hallmarks of ER-associated apoptosis.
Targeted Cell Death in Key Tissues
- Pancreatic Beta Cells: Chronic ER stress induces beta-cell apoptosis, significantly impairing insulin production. This is a primary cause of early-onset diabetes in individuals with Wolfram Syndrome.
- Neurons: In neural tissues, prolonged ER stress triggers apoptosis in retinal ganglion cells and cochlear neurons, leading to progressive vision and hearing loss.
Scientific Insight:
A pivotal study in the Journal of Clinical Investigation (Engin et al., 2013) highlighted the central role of ER-associated apoptosis in the degeneration of pancreatic beta cells due to WFS1 mutations.
This research emphasized that the failure of protective UPR mechanisms in Wolfram Syndrome contributes directly to cellular dysfunction and tissue degeneration.
The systemic implications of this process underline the devastating impact of unresolved ER stress, making it a critical factor in the progression of Wolfram Syndrome.
Systemic Effects of ER Dysfunction
Multisystem Degeneration:
The effects of WFS1 mutations extend beyond specific cell types, affecting multiple systems:
- Neurological: Progressive optic atrophy and hearing loss.
- Endocrine: Insulin deficiency and early-onset diabetes.
- Renal: Increased susceptibility to kidney dysfunction.
Link Between ER Dysfunction and Disease Progression:
Chronic ER stress and apoptosis contribute to the gradual decline in tissue function, exacerbating the systemic progression of Wolfram Syndrome.
Scientific Insight:
Research in Trends in Molecular Medicine (Hetz et al., 2012) emphasized the systemic consequences of unresolved ER stress, particularly in neurodegenerative and metabolic disorders.
Emily’s Battle with Diabetes and Hearing Loss
Emily, a 14-year-old diagnosed with Wolfram Syndrome, began her health challenges at age 7 with the onset of insulin-dependent diabetes.
By age 10, she started experiencing progressive hearing loss, which interfered with her ability to participate in school and social activities.
Genetic testing revealed a truncating mutation in the WFS1 gene, which disrupted endoplasmic reticulum (ER) calcium homeostasis.
This imbalance triggered chronic ER stress and apoptosis in cochlear neurons, leading to her hearing impairment.
Despite these challenges, early interventions with assistive hearing devices have helped Emily manage her symptoms.
Jacob’s Journey with Vision and Metabolic Challenges
Jacob, an 18-year-old college student, noticed blurred vision during his early teens, a symptom later identified as optic atrophy.
Coupled with recurrent episodes of hypoglycemia due to his insulin-dependent diabetes, Jacob’s condition was linked to Wolfram Syndrome.
Genetic investigations pinpointed a missense mutation in the WFS1 gene, which caused prolonged ER stress in retinal ganglion cells, leading to their degeneration.
Jacob’s case highlights how Wolfram Syndrome affects multiple systems, driven by WFS1 mutations that disrupt ER function.
Early diagnosis and symptom management have been key in maintaining Jacob’s quality of life during his academic pursuits.
FAQs on WFS1 Mutation and ER Stress in Wolfram Syndrome
Q-1: How do WFS1 mutations impair calcium regulation within the endoplasmic reticulum, contributing to ER dysfunction in Wolfram Syndrome?
A-1: WFS1 encodes wolframin, a protein integral to calcium homeostasis in the endoplasmic reticulum (ER). Mutations in WFS1 disrupt wolframin’s role in maintaining proper calcium levels inside the ER lumen.
This dysregulation leads to abnormal calcium depletion or overload, which impairs ER protein folding and chaperone activity. Calcium imbalance triggers ER stress and disturbs cellular signaling pathways, contributing to the progressive cell damage seen in pancreatic beta cells and neurons characteristic of Wolfram Syndrome.
Q-2: In what way do WFS1 mutations exacerbate the unfolded protein response (UPR) and contribute to chronic ER stress?
A-2: The unfolded protein response (UPR) is a protective mechanism activated when misfolded proteins accumulate in the ER. WFS1 mutations hinder the cell’s ability to resolve protein misfolding by impairing wolframin’s regulatory functions in ER homeostasis.
This leads to sustained UPR activation, which fails to restore normal function and instead shifts toward triggering apoptosis. The chronic ER stress caused by WFS1 mutations is a central driver of cellular loss in tissues affected by Wolfram Syndrome, including pancreatic islets and the nervous system.
Q-3: How do WFS1 mutations affect ER-mitochondria interactions and what are the consequences for cellular metabolism?
A-3: Wolframin encoded by WFS1 is involved in maintaining ER-mitochondria contact sites, which facilitate calcium transfer and energy metabolism coordination. Mutations in WFS1 disrupt these interactions, leading to impaired calcium signaling between the ER and mitochondria.
This causes mitochondrial dysfunction, decreased ATP production, and increased oxidative stress. The resulting energy deficit and cellular damage contribute to the neurodegeneration and beta-cell failure observed in Wolfram Syndrome.
Q-4: Can WFS1 mutations compromise ER-associated degradation (ERAD) pathways, and how does this impact cell survival?
A-4: Yes, WFS1 mutations can impair ER-associated degradation (ERAD), the process responsible for targeting misfolded proteins for degradation.
Dysfunctional wolframin reduces ERAD efficiency, causing accumulation of toxic misfolded proteins and aggravating ER stress. Persistent ER stress from impaired ERAD activates apoptotic signals, leading to progressive cell death and tissue degeneration in Wolfram Syndrome.
Q-5: How do WFS1 mutations interfere with ER calcium buffering capacity under cellular stress conditions?
A-5: Under stress, cells rely on ER calcium buffering to maintain protein folding and signaling functions. WFS1 mutations weaken wolframin’s ability to regulate calcium storage and release, reducing the ER’s buffering capacity. This leads to calcium leakage and destabilization of ER functions during stress, amplifying ER dysfunction and promoting apoptosis in vulnerable cell types.
Q-6: What role does altered ER membrane composition play in the dysfunction caused by WFS1 mutations?
A-6: Wolframin influences ER membrane integrity and lipid composition. Mutations in WFS1 can alter membrane lipid balance, affecting the fluidity and function of the ER membrane. These changes disrupt the localization and activity of ER-resident proteins critical for folding and calcium signaling, worsening ER stress and contributing to cellular degeneration in Wolfram Syndrome.
Q-7: How might WFS1 mutations affect the ER’s capacity to coordinate cellular stress responses beyond protein folding?
A-7: Beyond protein folding, the ER coordinates responses to oxidative stress, calcium signaling, and lipid metabolism. WFS1 mutations impair wolframin’s regulatory role in these pathways, diminishing the ER’s ability to orchestrate comprehensive stress responses. This broad dysfunction leads to increased cellular vulnerability, impaired adaptive mechanisms, and accelerated degeneration in tissues affected by Wolfram Syndrome.
Conclusion
Mutations in the WFS1 gene severely disrupt endoplasmic reticulum (ER) function, serving as a root cause of the progressive degeneration observed in Wolfram Syndrome.
Wolframin, the protein encoded by WFS1, plays a pivotal role in regulating protein folding, maintaining calcium homeostasis, and moderating the unfolded protein response (UPR).
When WFS1 mutations impair these functions, the ER becomes overwhelmed with stress, leading to chronic activation of stress pathways and cellular apoptosis.
The cascading effects include impaired insulin production due to beta-cell loss, neurodegeneration affecting hearing and vision, and a range of systemic dysfunctions.
Research in Nature Reviews Endocrinology (Fonseca et al., 2013) emphasized the centrality of ER stress in these processes, linking it directly to disease progression.
By uncovering these mechanisms, scientists and clinicians are gaining valuable insights into potential therapeutic approaches.
Advances in ER biology and molecular genetics hold promise for targeted treatments, offering hope for improved management and outcomes for individuals with Wolfram Syndrome.
Understanding these complex pathways is vital for developing innovative strategies to alleviate symptoms and decelerate the progression of this debilitating condition.
References: