How Obesity Affects the Genetic Predisposition to Alström Syndrome?
- admin
- January 13, 2025
- 1:13 pm
- No Comments
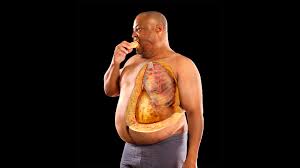
Obesity and Alström Syndrome are both complex conditions with significant genetic and environmental underpinnings.
Alström Syndrome is a rare autosomal recessive genetic disorder caused by mutations in the ALMS1 gene, leading to multisystem complications such as cardiomyopathy, type 2 diabetes, and progressive sensory impairments.
Obesity, often a hallmark of Alström Syndrome, can exacerbate its progression and severity by influencing gene expression and metabolic pathways.
BestDietarySupplementforDiabetics will explore how obesity interacts with the genetic predisposition to Alström Syndrome, highlighting mechanisms such as epigenetic changes, metabolic dysregulation, and oxidative stress.
Using scientific evidence and real-life examples, we aim to uncover the intricate relationship between obesity and Alström Syndrome.
Table of Contents
- Understanding Alström Syndrome and Obesity
- The Role of the ALMS1 Gene in Obesity and Metabolic Health
- Epigenetic Interactions Between Obesity and Genetic Predisposition
- Metabolic Pathways Amplified by Obesity
- 4.1 Insulin Resistance
- 4.2 Lipid Metabolism Dysregulation
- Inflammation and Oxidative Stress as Mediators
- Real-Life Examples
- 6.1 Emma’s Struggle with Weight and Diabetes
- 6.2 John’s Cardiac Challenges
- FAQs on Alström Syndrome and Obesity
- Conclusion
Understanding Alström Syndrome and Obesity
Alström Syndrome is a rare genetic disorder often marked by early-onset obesity, which typically begins in childhood.
This obesity is not just a symptom of the syndrome but an active driver of its progression, compounding the severity of related health issues.
Excess weight intensifies the metabolic challenges associated with Alström Syndrome, such as insulin resistance, type 2 diabetes, and cardiovascular complications.
Beyond its visible impact, obesity creates a state of chronic low-grade inflammation and hormonal imbalance, fostering conditions that worsen the disorder’s effects.
This inflammatory environment elevates levels of cytokines like interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α), which disrupt metabolic homeostasis and impair organ function.
Additionally, hormonal imbalances triggered by obesity, such as elevated leptin and reduced adiponectin levels, further strain the body’s ability to regulate energy and glucose.
Together, these factors amplify the phenotypic expression of Alström Syndrome, accelerating disease progression and complicating management strategies.
The Role of the ALMS1 Gene in Obesity and Metabolic Health
The ALMS1 gene encodes a protein critical for cellular functions such as ciliary activity, intracellular trafficking, and energy regulation.
These processes are fundamental to maintaining metabolic balance and normal cellular operations. Mutations in ALMS1 disrupt these pathways, impairing glucose metabolism and lipid regulation, two processes intricately tied to body weight.
Obesity exacerbates these dysfunctions by overloading metabolic pathways with excess energy demands, intensifying the strain on systems already compromised by ALMS1 mutations.
This creates a feedback loop where metabolic imbalances further worsen obesity and its associated complications.
Supporting Evidence
A study in Nature Genetics (Marshall et al., 2005) found that ALMS1-deficient cells exhibited severe abnormalities in energy homeostasis and lipid accumulation.
This research highlighted how mutations in the gene directly contribute to dysregulated fat storage and insulin signaling, intensifying metabolic challenges.
Obesity compounds these issues, accelerating the progression of systemic complications like diabetes and cardiovascular disease in individuals with Alström Syndrome.
Epigenetic Interactions Between Obesity and Genetic Predisposition
Obesity triggers epigenetic modifications, such as DNA methylation and histone acetylation, that influence gene expression without altering the genetic code itself.
In individuals with Alström Syndrome, these modifications may amplify the harmful phenotypes associated with ALMS1 mutations increases insulin resistance thereby worsening metabolic and systemic dysfunctions.
Key Mechanisms:
- DNA Methylation:
Obesity is associated with increased methylation of key metabolic genes, silencing protective pathways critical for glucose and lipid regulation. In Alström Syndrome, this can exacerbate pre-existing metabolic imbalances linked to ALMS1 mutations. - Histone Modification:
Obesity-induced histone acetylation alters the accessibility of genes involved in energy homeostasis. This compounds challenges in cellular energy regulation and lipid storage, central issues in Alström Syndrome.
Scientific Insight:
A study in Epigenetics (Steegenga et al., 2014) found that obesity-induced epigenetic changes heightened the severity of genetic predispositions in metabolic disorders.
This suggests that obesity may accelerate the progression of Alström Syndrome by modifying the epigenetic landscape, increasing the expression of deleterious phenotypes and further impairing cellular functions.
These findings highlight the complex interplay between obesity and genetic disorders, emphasizing the need to manage weight in individuals with Alström Syndrome.
Metabolic Pathways Amplified by Obesity
Let us see through this point in brief:
Insulin Resistance:
Obesity is a major driver of insulin resistance, characterized by reduced cellular responsiveness to insulin. In Alström Syndrome, where insulin resistance is already present due to ALMS1 mutations, obesity accelerates the onset of type 2 diabetes.
Supporting Evidence:
Research in The Journal of Clinical Endocrinology & Metabolism (Eisenberger et al., 2012) found that nearly all Alström Syndrome patients with obesity developed diabetes by their teenage years, underscoring the interplay between genetic and environmental factors.
Lipid Metabolism Dysregulation:
The ALMS1 protein plays a role in lipid transport and storage. Obesity amplifies lipid dysregulation, leading to ectopic fat deposition in organs such as the liver and pancreas, further impairing their function.
Example:
John, a 30-year-old with Alström Syndrome, experienced worsening fatty liver disease due to uncontrolled obesity. His medical team highlighted the need for weight management to alleviate hepatic strain.
Inflammation and Oxidative Stress as Mediators
Obesity induces chronic low-grade inflammation, characterized by elevated levels of pro-inflammatory cytokines like tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6).
These cytokines exacerbate systemic inflammation, which can cross the blood-brain barrier, leading to neuroinflammation and aggravating complications associated with Alström Syndrome such as hearing loss.
The persistent inflammatory state disrupts normal cellular processes, worsening metabolic and systemic symptoms such as diabetes and cardiovascular dysfunction.
Oxidative Stress and Mitochondrial Damage
Excess fat mass in obesity increases the production of reactive oxygen species (ROS), molecules that cause oxidative damage to proteins, lipids, and DNA.
ROS generation leads to mitochondrial dysfunction, a hallmark of Alström Syndrome driven by ALMS1 mutations.
When mitochondrial function is already impaired, as seen in Alström Syndrome, obesity-induced oxidative stress amplifies cellular damage, accelerating disease progression.
Scientific Insight:
A study published in Obesity Reviews (Fardet et al., 2016) highlighted that chronic inflammation and oxidative stress associated with obesity accelerate disease progression in individuals with underlying genetic predispositions.
For those with Alström Syndrome, these mechanisms deepen metabolic dysregulation and cellular impairments, making obesity a critical modifier of disease severity.
Addressing inflammation and oxidative stress is crucial for mitigating the compounded effects of obesity in Alström Syndrome patients.
Emma’s Battle with Obesity and Early-Onset Diabetes
Emma, an 8-year-old diagnosed with Alström Syndrome, began gaining weight rapidly at just five years old.
Her parents initially attributed her weight gain to a growth spurt, but the persistence of her symptoms prompted further investigation.
By the age of 10, Emma had developed type 2 diabetes, a common metabolic complication in Alström Syndrome, exacerbated by her early-onset obesity.
Emma’s doctors emphasized a specialized intervention plan, using a glucometer, including a low-glycemic diet, tailored to manage her blood sugar levels, and physical therapy to encourage regular physical activity without overexertion.
Despite these measures stabilizing her glucose levels, Emma’s genetic predisposition to insulin resistance meant that her struggle was ongoing.
Her journey highlights the compounding effects of obesity on inherited metabolic dysfunctions.
John’s Cardiac Setback
John, a 15-year-old with Alström Syndrome, developed obesity by age 12, further complicating his pre-existing cardiomyopathy.
His excessive weight placed additional strain on his heart, worsening symptoms such as fatigue, shortness of breath, and reduced cardiac output.
John’s medical team implemented a multifaceted treatment approach, including weight loss strategies, cardiac medications, and moderate exercise tailored to his capabilities.
While these interventions showed slight improvement in his heart function, the compounded effects of obesity and Alström Syndrome remained a challenge, showcasing the need for early lifestyle interventions.
Both Emma’s and John’s stories illustrate how obesity intensifies Alström Syndrome symptoms, underscoring the importance of holistic, proactive management.
FAQs on Alström Syndrome and Obesity
Q-1: How does obesity influence the severity of metabolic complications in individuals with Alström syndrome?
A-1: Obesity in Alström syndrome can exacerbate metabolic complications by increasing insulin resistance, leading to higher blood glucose levels and a greater risk of developing type 2 diabetes. Additionally, obesity may contribute to hypertriglyceridemia and hypertension, further complicating the metabolic profile of affected individuals.
Q-2: Does obesity affect the progression of cardiomyopathy in Alström syndrome patients?
A-2: Yes, obesity can accelerate the progression of cardiomyopathy in Alström syndrome. The increased adiposity places additional strain on the heart, potentially leading to more severe cardiac dysfunction and a higher risk of heart failure.
Q-3: How does obesity impact the endocrine abnormalities associated with Alström syndrome?
A-3: Obesity can worsen endocrine abnormalities in Alström syndrome, such as hypothyroidism and hypogonadism. Excess weight may exacerbate insulin resistance, leading to higher insulin levels and further disrupting hormonal balance.
Q-4: What is the relationship between obesity and hearing loss in Alström syndrome?
A-4: While obesity does not directly cause hearing loss in Alström syndrome, it can contribute to overall health deterioration, potentially affecting auditory function. The systemic effects of obesity may indirectly influence the progression of hearing impairment.
Q-5: How does obesity affect the management and treatment of Alström syndrome?
A-5: Obesity complicates the management of Alström syndrome by necessitating more intensive interventions to control metabolic parameters. Weight management becomes a critical component of treatment to mitigate obesity-related complications and improve overall health outcomes.
Q-6: Are there any interactions between obesity and vision problems in Alström syndrome?
A-6: Obesity may indirectly affect vision problems in Alström syndrome by contributing to metabolic disturbances that can impact retinal health. However, the primary cause of vision issues in Alström syndrome is retinal degeneration, and obesity’s role is secondary.
Q-7: How does obesity influence the progression of liver disease in Alström syndrome?
A-7: Obesity can exacerbate liver disease in Alström syndrome by increasing the risk of non-alcoholic fatty liver disease (NAFLD). The accumulation of fat in the liver can lead to inflammation and fibrosis, potentially progressing to cirrhosis and liver failure.
Takeaway
Obesity exacerbates the genetic predisposition to Alström Syndrome by intensifying metabolic imbalances, amplifying inflammatory responses, and accelerating disease progression.
Individuals with Alström Syndrome already face challenges such as insulin resistance, cardiomyopathy, and lipid dysregulation due to mutations in the ALMS1 gene, and obesity compounds these issues.
Chronic inflammation, a hallmark of obesity, elevates levels of pro-inflammatory cytokines like TNF-α and IL-6, which worsen systemic complications.
Additionally, obesity increases oxidative stress, further impairing mitochondrial function, which is already compromised in Alström Syndrome.
Scientific studies, such as one published in Obesity Reviews (Fardet et al., 2016), highlight the role of obesity-induced inflammation in worsening inherited disorders.
Understanding how obesity interacts with ALMS1 mutations offers new opportunities for targeted interventions.
Lifestyle modifications tailored to reduce weight and inflammation, combined with ongoing research into genetic therapies, could significantly improve the prognosis for affected individuals.
This dual approach underscores the importance of addressing obesity in the management of Alström Syndrome.
References: