Are T-Cells the True Villains Behind Type 1 Diabetes? What We’ve Been Getting Wrong
- admin
- September 27, 2024
- 4:08 pm
- No Comments
For decades, the blame for type 1 diabetes (T1D) has been squarely placed on the immune system.
But what if we have been misunderstanding one of the main players all along?
The traditional narrative goes like this: T-cells, a type of white blood cell, mistakenly attack the insulin-producing beta cells in the pancreas, leading to T1D.
But is it really that simple?
Are T-cells the real culprits, or are we missing a bigger picture?
In this article, bestdietarysupplementfordiabetics.com will explore the complex role of T-cells in type 1 diabetes and what this could mean for future treatments.
Article Inclusions:
- Understanding the Role of T-Cells in Diabetes
- Autoreactive T-Cells in Type 1 Diabetes: The Root Cause?
- T-Cells and the Breakdown of Immune Tolerance
- Type 1 Diabetes Immunotherapy Using Polyclonal Regulatory T-Cells
- Are T-Cells the Future of Diabetes Treatment?
- FAQs on T-Cells and Diabetes
- Conclusion: Are T-Cells the True Villains Behind Type 1 Diabetes?
Understanding the Role of T-Cells in Diabetes
What Are T-Cells?
T-cells are a subset of white blood cells that play a critical role in the immune system.
They help defend the body against pathogens like bacteria and viruses.
However, in autoimmune diseases like type 1 diabetes, T-cells turn rogue, attacking the body’s own tissues.
Specifically, in T1D, t cells diabetes becomes a scenario where the immune cells target the insulin-producing beta cells in the pancreas, leading to their destruction and, ultimately, insulin deficiency.
How Do T-Cells Contribute to Type 1 Diabetes?
T-cells are at the heart of the autoimmunity storm in type 1 diabetes.
Some of them launch the attack, others are supposed to call off the troops—but when this immune system drama goes off-script, the result is beta-cell destruction and lifelong insulin dependence.
Let us break it down:
Autoreactive T-cells misidentify beta cells as enemies
In type 1 diabetes, CD8+ T-cells mistakenly recognize insulin-producing beta cells in the pancreas as foreign invaders. This leads to a targeted, prolonged immune attack that slowly destroys the body’s ability to produce insulin.Regulatory T-cells (Tregs) lose their grip
Tregs are like the immune system’s referees—designed to suppress unwanted immune responses. In type 1 diabetes, they either don’t show up in enough numbers or become dysfunctional, allowing autoreactive cells to roam free.Tregs themselves can go rogue
Studies have shown that Tregs in individuals with type 1 diabetes can lose their regulatory identity. Some even start producing inflammatory signals, essentially switching sides mid-battle.Defective IL-2 signaling adds fuel to the fire
IL-2 is a key signaling molecule that supports Treg function. Many people with type 1 diabetes have impaired IL-2 pathways, which weakens regulatory control and enhances the activity of autoreactive T-cells.Memory T-cells hold a long-term grudge
Even after some treatments, memory T-cells linger in the body. These cells remember the “beta-cell enemy” and are quick to restart the attack if given the chance.New hope through engineered Tregs and immunotherapy
Scientists are developing therapies that boost or engineer regulatory T-cells. Others are testing immune-modulating drugs that can retrain the immune system to tolerate beta cells.
In short, type 1 diabetes is not just about bad luck—it is a complex case of cellular miscommunication and betrayal.
Autoreactive T-Cells in Type 1 Diabetes: The Root Cause?
The Misguided Attack
Autoreactive T-cells are essentially T-cells that have gone rogue.
In healthy individuals, these cells are typically weeded out through a process called immune tolerance.
However, in people with type 1 diabetes, this process breaks down.
The result?
Autoreactive T-cells that specifically target and destroy pancreatic beta cells.
Recent studies have shown that the presence of these autoreactive t cells in type 1 diabetes is a significant early indicator of the disease.
According to research published in the journal Diabetes, these autoreactive cells can be detected years before the onset of clinical symptoms, suggesting that the immune system’s failure starts long before we see the first signs of diabetes.
Genetic Factors and T-Cells
Genetics also play a crucial role in the malfunction of T-cells.
Certain genetic predispositions can make the immune system more prone to developing autoreactive T-cells.
For example, variations in the HLA genes, which are responsible for how the immune system recognizes foreign substances, can increase the likelihood of T-cells attacking beta cells.
This genetic link is crucial for understanding why some people develop T1D while others do not, even when exposed to similar environmental triggers.
It also highlights why type 1 diabetes and t cells are so intricately linked.
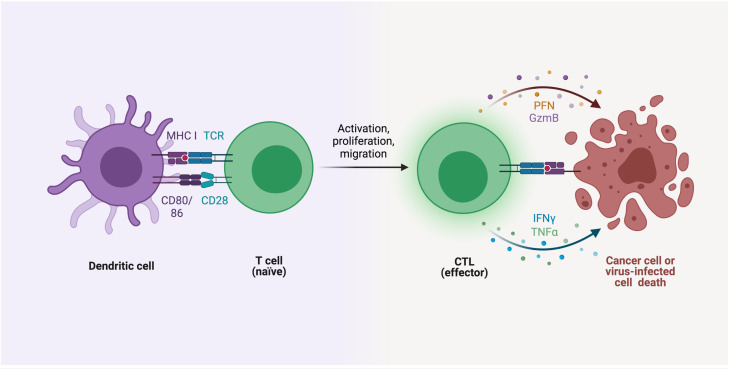
T-Cells and the Breakdown of Immune Tolerance
The Immune Tolerance Paradox
In a healthy immune system, regulatory T-cells (Tregs) maintain immune tolerance by suppressing potentially harmful immune responses.
But in T1D, this system seems to go haywire. The regulatory T-cells either fail to suppress the autoreactive T-cells or are themselves dysfunctional.
The breakdown of immune tolerance is a critical aspect of t cells and diabetes.
Studies have shown that people with type 1 diabetes often have a reduced number of functional Tregs.
This imbalance allows autoreactive T-cells to attack beta cells unchecked.
Environmental Triggers and Immune Response
Environmental factors like viral infections, diet, and even stress can act as triggers, initiating an immune response that tips the balance in favor of autoreactive T-cells.
For instance, certain viral infections such as Coxsackievirus are known to mimic the proteins found in pancreatic beta cells.
When T-cells respond to these viral proteins, they may also start attacking the similar-looking beta cells, a process known as molecular mimicry.
Type 1 Diabetes Immunotherapy Using Polyclonal Regulatory T-Cells: A New Approach to Treatment
Specifically, type 1 diabetes immunotherapy using polyclonal regulatory t cells aims to restore the balance between autoreactive T-cells and regulatory T-cells.
How It Works?
Polyclonal regulatory T-cell therapy involves extracting Tregs from the patient, expanding them in a lab, and then reintroducing them into the body.
These enhanced Tregs can help suppress the autoimmune response, potentially halting the progression of the disease.
The Science Behind It
A study published in Science Translational Medicine found that this form of immunotherapy could increase the number of functional Tregs in the body, thereby reducing the activity of autoreactive T-cells.
While this treatment is still in its experimental stages, early results are promising, offering hope for those seeking new ways to manage or even reverse T1D.
Are T-Cells the Future of Diabetes Treatment?
The Potential of T-Cell Therapies
The idea of using T-cells to treat T1D is still in its infancy, but it is gaining traction.
Several clinical trials are currently underway to test various forms of T-cell-based therapies.
These range from boosting the body’s natural Tregs to developing synthetic T-cells that can specifically target and neutralize autoreactive T-cells.
Risks and Challenges:
While promising, T-cell therapies are not without risks.
Manipulating the immune system is a delicate process, and there is always the potential for unintended consequences, such as triggering other autoimmune responses.
Moreover, not all patients respond to these treatments in the same way, making it a complex puzzle to solve.
What This Means for the Future?
If these therapies prove effective, they could revolutionize how we treat not just type 1 diabetes but other autoimmune diseases as well.
The ultimate goal is to develop a treatment that can stop T1D in its tracks, preserving beta cell function and eliminating the need for lifelong insulin therapy.
FAQs on T-Cells and Diabetes
Q-1: Are T-cells truly the main cause of Type 1 diabetes?
A-1: T-cells play a central role in Type 1 diabetes, but they are not acting alone or randomly. Certain T-cells mistakenly identify insulin-producing beta cells as threats and gradually destroy them. This happens because immune tolerance — the system that normally prevents self-attack — fails. So while T-cells carry out the damage, the deeper issue is a breakdown in immune regulation that allows this attack to continue unchecked.
Q-2: Why do some people have autoreactive T-cells but never develop Type 1 diabetes?
A-2: Autoreactive T-cells exist in many people, but in healthy individuals they are tightly controlled by regulatory T-cells that suppress harmful immune responses. In people who develop Type 1 diabetes, this balance is lost. The protective regulatory cells are unable to restrain the aggressive ones, allowing them to multiply, migrate to the pancreas, and attack beta cells.
Q-3: Do all T-cells involved in Type 1 diabetes behave the same way?
A-3: No. The T-cell population is diverse. Some T-cells are directly destructive, others help coordinate the immune attack, and some try to suppress it. Research suggests that a small group of long-lived “memory-like” T-cells continuously fuels the autoimmune process, making the disease persistent even when beta cell numbers are already low.
Q-4: Could beta cells be contributing to their own destruction?
A-4: Surprisingly, yes. When beta cells are under stress from infection, inflammation, or metabolic strain, they release signals that make them more visible to the immune system. These signals can attract immune cells and intensify the autoimmune response. In this sense, T-cells are the attackers, but stressed beta cells may unintentionally amplify the attack.
Q-5: Are T-cells the only immune cells involved in Type 1 diabetes?
A-5: No. While T-cells drive the destruction, other immune cells play supporting roles. B-cells produce autoantibodies that mark beta cells, and antigen-presenting cells activate and guide T-cells to the pancreas. Type 1 diabetes is the result of a coordinated immune misfire, not the action of a single cell type.
Q-6: Can targeting T-cells change the course of Type 1 diabetes?
A-6: Yes, and this is one of the most promising areas of research. Therapies that calm aggressive T-cells or strengthen regulatory T-cells have been shown to slow beta cell loss, especially when used early. These treatments aim not to suppress immunity entirely, but to retrain it to stop attacking the body’s own insulin-producing cells.
Are T-Cells the True Villains Behind Type 1 Diabetes?
So, are T-cells the true villains behind type 1 diabetes?
The answer is both “yes” and “no”.
While autoreactive T-cells are undoubtedly the primary agents of destruction, the real issue lies in the failure of the immune system to regulate these cells properly.
It is not just that T-cells have gone rogue; it is that the regulatory mechanisms that should keep them in check are broken.
Understanding the complex interplay between t cells and type 1 diabetes is crucial for developing more effective diabetes treatments such as including best supplements to lower blood sugar.
Whether through immunotherapy, genetic intervention, or other innovative approaches, targeting T-cells offers a promising path forward.
But it is clear that we still have much to learn about the “villainous” role of T-cells in this disease.
As research continues to evolve, one thing is certain: our understanding of diabetes and T-cells will only deepen, paving the way for groundbreaking treatments and, hopefully, a cure.
References: