How Beta-Cell Mutations Cause MODY Diabetes?
- admin
- December 1, 2024
- 5:50 pm
- No Comments
Monogenic diabetes, commonly known as MODY (Maturity-Onset Diabetes of the Young), is a rare form of diabetes caused by mutations in specific genes.
Unlike the more prevalent forms of diabetes, MODY is linked directly to beta-cell dysfunction due to genetic mutations.
This article by bestdietarysupplementfordiabetics.com explores how mutations in beta cells contribute to MODY diabetes, delving into the molecular mechanisms, types of mutations, and their impacts.
By examining scientific studies, we aim to understand the role of beta-cell mutations in this condition.
Table of Contents:
- Introduction to MODY Diabetes
- The Role of Beta Cells in Insulin Secretion
- Genetic Foundations of MODY
- Key Beta-Cell Mutations and Their Effects
- HNF1A Mutations (MODY3)
- GCK Mutations (MODY2)
- HNF4A Mutations (MODY1)
- Mechanisms of Beta-Cell Dysfunction
- Real-Life Case Study: David’s MODY Diagnosis
- Diagnosis and Genetic Testing
- FAQs
- Conclusion
Introduction to MODY Diabetes
MODY is a rare and often misdiagnosed form of diabetes caused by a single gene mutation that disrupts the function of pancreatic beta cells.
Unlike Type 1 or Type 2 diabetes, MODY is inherited in an autosomal dominant pattern.
This article focuses on the link between beta-cell mutations and their role in MODY, exploring molecular mechanisms and real-world implications.
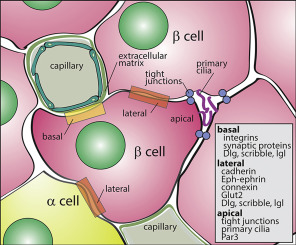
The Role of Beta Cells in Insulin Secretion
Beta cells, found in the islets of Langerhans within the pancreas, play a critical role in maintaining glucose homeostasis.
They produce and secrete insulin, a hormone essential for enabling cells to absorb glucose from the bloodstream.
This process ensures stable blood sugar levels and provides energy for the body’s metabolic activities.
Beta cells are highly specialized, relying on precise genetic instructions to sense glucose and respond by releasing the appropriate amount of insulin.
These functions are tightly regulated by genes like HNF1A, GCK, and HNF4A, which ensure that beta cells work efficiently.
When mutations occur in these genes, the delicate balance of insulin production is disrupted.
This dysfunction reduces insulin secretion, even when blood glucose levels rise, leading to impaired glucose regulation—a hallmark of MODY diabetes.
Understanding the genetic link between beta-cell mutations and MODY is crucial for diagnosing and managing this rare condition.
Genetic Foundations of MODY
MODY (Maturity-Onset Diabetes of the Young) is a monogenic form of diabetes caused by mutations in at least 14 identified genes, with HNF1A, GCK, and HNF4A being the most commonly implicated.
These mutations disrupt critical beta-cell processes, such as glucose sensing, insulin gene expression, and secretion.
For example, mutations in HNF1A impair the beta cell’s ability to regulate insulin production, while GCK mutations affect glucose sensing, leading to stable but elevated blood sugar levels.
Unlike Type 1 diabetes, MODY does not involve autoimmune beta-cell destruction.
Similarly, it differs from Type 2 diabetes because it is not linked to insulin resistance or obesity.
MODY’s genetic basis makes it an inherited condition, with an autosomal dominant inheritance pattern, meaning a 50% chance of passing the condition to offspring.
Understanding these genetic foundations allows for early diagnosis and tailored treatments, such as sulfonylureas for some subtypes.
Key Beta-Cell Mutations and Their Effects:
HNF1A Mutations (MODY3)
HNF1A, a transcription factor, regulates genes critical for beta-cell function.
Mutations in this gene result in reduced insulin secretion, even though beta cells are structurally intact. Individuals with MODY3 often develop diabetes in adolescence or early adulthood, characterized by mild hyperglycemia.
Study Insight: Research published in the Journal of Clinical Endocrinology & Metabolism revealed that HNF1A mutations lead to a 50% reduction in beta-cell function by the mid-20s.
GCK Mutations (MODY2)
GCK, or glucokinase, acts as a glucose sensor in beta cells. Mutations in the GCK gene result in an elevated glucose threshold for insulin secretion, causing mild, stable hyperglycemia that is often asymptomatic.
Unlike MODY3, MODY2 typically does not require pharmacological intervention.
Study Insight: A study in Diabetologia showed that individuals with GCK mutations rarely develop complications, underscoring the gene’s role in glucose sensing rather than insulin production.
HNF4A Mutations (MODY1)
HNF4A mutations disrupt both insulin production and beta-cell differentiation.
This subtype often presents with severe hyperglycemia and can mimic the symptoms of Type 1 diabetes.
Study Insight: According to research in Nature Genetics, HNF4A mutations also affect lipid metabolism, contributing to broader metabolic dysfunctions in MODY1.
Beta-cell dysfunction is the central mechanism by which MODY diabetes develops. This dysfunction arises due to genetic mutations that interfere with insulin secretion and glucose regulation.
Here are the primary mechanisms:
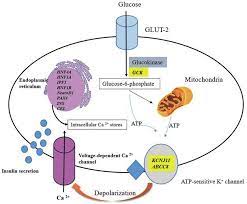
Impaired Glucose Sensing:
Beta cells rely on glucose sensing to determine when insulin release is required. Mutations in the GCK gene, which encodes glucokinase, disrupt this process.
Glucokinase acts as a glucose sensor, setting the threshold for insulin secretion.
Mutations in this gene lead to an altered glucose threshold, where beta cells fail to respond adequately to rising blood sugar levels. This results in stable but consistently elevated blood glucose levels, characteristic of MODY2.
Reduced Insulin Gene Expression:
Transcription factors such as HNF1A and HNF4A play critical roles in regulating insulin gene expression.
Mutations in these genes impair transcriptional processes, reducing insulin mRNA levels and subsequent insulin production.
This defect is prominent in MODY1 and MODY3, where insufficient insulin secretion leads to postprandial hyperglycemia and long-term glucose intolerance.
Beta-Cell Exhaustion:
As beta cells attempt to compensate for reduced insulin production, they become overworked.
Over time, this leads to progressive beta-cell failure, further reducing insulin output.
This is particularly evident in MODY3, where early interventions can delay this progression.
Scientific Validation:
A study published in Diabetes Care highlighted that beta-cell exhaustion is a key factor in MODY progression, often necessitating pharmacological intervention such as sulfonylureas.
This emphasizes the importance of early diagnosis and treatment in preserving beta-cell function.
David’s MODY Diagnosis
David, a 25-year-old college student, discovered he had elevated blood sugar levels during a routine health screening.
With a family history of diabetes, his doctor initially diagnosed him with Type 1 diabetes and started him on insulin therapy.
However, David’s symptoms were atypical—he showed no signs of insulin resistance or autoimmunity.
Further evaluation and genetic testing revealed a mutation in the HNF1A gene, confirming a diagnosis of MODY3.
This finding changed his treatment approach significantly.
David’s endocrinologist prescribed sulfonylureas, oral medications that enhance insulin secretion by stimulating beta cells.
Within weeks, David achieved stable glycemic control without the need for insulin injections.
David’s experience underscores the importance of understanding beta-cell mutations as the root cause of MODY.
Accurate diagnosis not only prevents unnecessary treatments but also enables personalized management strategies, improving long-term outcomes.
His story also highlights the value of genetic testing in differentiating MODY from other forms of diabetes.
Diagnosis and Genetic Testing
Genetic testing is crucial for accurately diagnosing MODY, utilizing advanced techniques like Next-Generation Sequencing (NGS) to detect mutations in MODY-associated genes such as HNF1A, GCK, and HNF4A.
This precise approach offers several key benefits:
- Accurate Diagnosis: Differentiates MODY from Type 1 and Type 2 diabetes, which often have overlapping symptoms but distinct causes and treatment needs.
- Personalized Treatment: Identifying specific genetic mutations helps guide therapy. For instance, MODY3 patients with HNF1A mutations often respond well to sulfonylureas instead of insulin.
- Family Screening: Genetic testing identifies at-risk relatives, enabling early interventions to prevent complications or delays in management.
Study Insight:
Research published in Endocrine Reviews emphasized that early genetic testing significantly improves outcomes by preventing misdiagnoses and unnecessary treatments.
It allows healthcare providers to design tailored management plans, ensuring patients receive appropriate care aligned with their genetic profiles.
FAQs on Beta Cell Mutation & MODY
Q-1: What beta-cell defects turn a single gene glitch into lifelong dysglycemia in MODY?
A-1: MODY mutations typically hit one of four β-cell “jobs”: (1) glucose sensing (e.g., GCK) shifts the set-point so insulin release starts at higher glucose; (2) protein folding/processing (e.g., INS) misfolds proinsulin, provoking ER stress and reducing healthy insulin output; (3) stimulus–secretion coupling (e.g., KCNJ11/ABCC8 KATP channels) blunts membrane depolarization and calcium entry; and (4) identity/transcription control (e.g., HNF1A, HNF4A, PDX1) lowers the machinery that drives insulin gene expression and granule release.
Q-2: Why do some MODY types stay mild for decades while others progress?
A-2: It depends on where the defect sits. GCK-MODY mainly resets the “thermostat,” so hyperglycemia is mild and stable. Transcription-factor or KATP variants progressively reduce β-cell reserve, so post-meal spikes enlarge and fasting glucose creeps up with age. INS misfolding can accelerate loss by stressing and depleting β-cells, leading to earlier need for medication or insulin.
Q-3: Which lab clues hint that a β-cell mutation—not type 1 or type 2—is driving the hyperglycemia?
A-3: Hallmarks include autoantibody-negative hyperglycemia in youth or young adulthood, autosomal-dominant family history across generations, and preserved C-peptide beyond the honeymoon period. Pattern specifics help: lifelong, modest fasting elevation suggests GCK; striking sensitivity to tiny doses of sulfonylureas points toward HNF1A/HNF4A; a high proinsulin-to-insulin (or C-peptide) ratio hints at INS misprocessing.
Q-4: How do genotype–therapy matches change real-world care?
A-4: In GCK-MODY, medication is often unnecessary outside pregnancy because complications risk is low. HNF1A/HNF4A forms typically respond dramatically to low-dose sulfonylureas or short-acting secretagogues, sometimes replacing insulin. KATP-channel variants may also respond to sulfonylureas. INS-related disease is variable; the guiding principle is to minimize β-cell secretory strain—steady targets, protein- and fiber-forward meals, and early consideration of basal or prandial insulin if progression appears.
Q-5: How does confirming a β-cell mutation change family planning and screening?
A-5: Most MODY types are autosomal dominant: each child has about a 50% chance of inheriting the variant. A genetic result clarifies who needs early, periodic glucose checks (often starting in late childhood), which therapies to try first if dysglycemia emerges, and how to manage pregnancy: GCK-MODY requires tailored targets because fetal genotype affects growth, while HNF-type MODY often benefits from planned secretagogue use with careful hypoglycemia monitoring.
Takeaway: MODY is a β-cell problem with many faces—glucose sensing, protein folding, stimulus–secretion coupling, or transcriptional identity. Recognizing the pattern steers testing and unlocks targeted therapy: often “no drugs” for GCK, secretagogue responsiveness for HNF/KATP forms, and stress-minimizing strategies (sometimes insulin) for INS. Genetic confirmation then maps who else in the family needs smart, earlier surveillance.
Conclusion
Beta-cell mutations are at the core of MODY diabetes, directly affecting insulin secretion and glucose regulation.
By understanding the genetic underpinnings, clinicians can make accurate diagnoses and provide tailored treatment options.
Genetic testing plays a critical role in identifying MODY, enabling early intervention and better management strategies.
Addressing beta-cell dysfunction not only improves patient outcomes but also provides insights into the broader field of diabetes research.
For individuals like David, understanding the genetic basis of their condition has been transformative, allowing for effective management and improved quality of life.
References: