As per BestDietarySupplementfordDiabetics research team, “Chronic inflammation is like that uninvited guest at your party who refuses to leave, causing more and more chaos the longer they stick around”.
But when it comes to your body, the consequences are much more severe than a trashed living room.
Chronic inflammation does not just make you feel lousy—it can wreak havoc on your organs, including your pancreas.
And that is where our topic today comes in: How chronic inflammation triggers type 3c diabetes, a lesser-known but very real type of diabetes that often gets overshadowed by type 1 and type 2.
In this article, we will explore the science behind how persistent inflammation can lead to pancreatic damage and, eventually, type 3c diabetes.
We will break it down into easily digestible parts, discuss some real-life examples, and back up everything with solid research.
Let’s dive in!
Article Index
- What Is Type 3c Diabetes?
- Understanding Chronic Inflammation
- How Inflammation Damages the Pancreas
- The Role of Pancreatitis in Type 3c Diabetes
- How Chronic Inflammation Affects Insulin Production
- FAQs on Chronic Inflammation & Type 3c Diabetes
- Real-Life Example: When Chronic Pancreatitis Leads to Diabetes
- The Science Behind Inflammatory Markers and Pancreatic Damage
- Conclusion: Understanding the Inflammation-Diabetes Connection
What Is Type 3c Diabetes?
Before we dive into inflammation, let’s clear up what type 3c diabetes actually is.
Also known as “pancreatogenic diabetes,” type 3c diabetes occurs when a condition or damage affecting the pancreas leads to impaired insulin production.
Unlike type 1 diabetes, where the immune system attacks insulin-producing cells, or type 2, which is usually linked to lifestyle factors, type 3c is primarily caused by pancreatic disorders such as chronic pancreatitis, cystic fibrosis, or pancreatic cancer.
According to a study published in Diabetes Care, type 3c diabetes is often underdiagnosed or misclassified as type 2 diabetes, making its management even more challenging.
Understanding Chronic Inflammation
Inflammation is your body’s natural response to injury or infection.
Think of it as your immune system’s way of sounding the alarm, sending out white blood cells and inflammatory chemicals to tackle the problem.
This is great in the short term, like when you have a minor cut or a cold.
But when inflammation becomes chronic, it is like your immune system stays in battle mode indefinitely, even when there is no real threat.
Dr. Charles Serhan’s research at Harvard University describes how chronic inflammation disrupts the normal functioning of tissues and organs, setting the stage for diseases like diabetes.
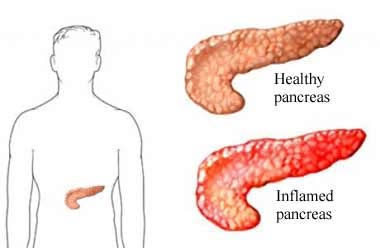
How Inflammation Damages the Pancreas?
Now, how does chronic inflammation specifically affect your pancreas?
The pancreas is a crucial organ for digestion and insulin regulation, and inflammation can damage both its exocrine (digestive enzyme-producing) and endocrine (hormone-producing) parts.
Persistent inflammation can cause scarring (fibrosis) and destroy the cells responsible for insulin production, known as beta cells.
A 2018 study in The Journal of Clinical Investigation revealed that prolonged exposure to inflammatory markers, such as cytokines, accelerates the death of beta cells, leading to reduced insulin output.
Imagine your pancreas as a factory producing insulin. Chronic inflammation is like a series of small fires that keep breaking out, destroying machinery and slowing down production until, eventually, the factory cannot keep up with demand.
The Role of Pancreatitis in Type 3c Diabetes
One of the main culprits connecting inflammation and type 3c diabetes is pancreatitis.
Chronic pancreatitis is a condition where inflammation of the pancreas becomes persistent, causing irreversible damage.
Over time, the repeated inflammation leads to fibrosis, which severely disrupts the pancreas’s ability to produce insulin.
According to Gastroenterology, nearly 80% of people with chronic pancreatitis will develop some form of glucose intolerance, and a significant percentage will progress to full-blown type 3c diabetes.
The more severe the pancreatitis, the higher the risk.
How Chronic Inflammation Affects Insulin Production?
So, what happens when chronic inflammation keeps beating up on your pancreas?
The beta cells become exhausted and die off, meaning they produce less insulin.
And without enough insulin, your body struggles to manage blood sugar levels effectively.
This is the essence of how type 3c diabetes develops.
The American Journal of Pathology published a study showing that inflammatory markers like interleukin-1 beta (IL-1β) are particularly harmful to beta cells.
These inflammatory chemicals make the cells less efficient at producing insulin and can even trigger cell death through a process called apoptosis.
When Chronic Pancreatitis Leads to Diabetes?
Consider the case of Nora, a 45-year-old woman who was diagnosed with chronic pancreatitis after years of alcohol misuse.
At first, she only experienced digestive issues, but over time, she started noticing symptoms of diabetes: increased thirst, frequent urination, and fatigue.
Her doctors eventually diagnosed her with type 3c diabetes.
Nora’s story highlights a key point: chronic inflammation does not just cause discomfort; it can lead to permanent damage and life-altering conditions like type 3c diabetes.
FAQs on Chronic Inflammation & Type 3c Diabetes
The Science Behind Inflammatory Markers and Pancreatic Damage
Inflammatory markers like cytokines, tumor necrosis factor-alpha (TNF-α), and interleukins play a significant role in damaging pancreatic tissue.
These markers are like little chemical messengers that signal the immune system to stay on high alert.
While this is helpful in fighting infections, chronic exposure to these markers can destroy healthy tissue.
A study in Nature Reviews Endocrinology found that high levels of TNF-α were linked to increased pancreatic cell apoptosis and insulin resistance, two key factors in the development of type 3c diabetes.
In simpler terms, the more inflammation, the higher the risk of severe pancreatic dysfunction.
Understanding the Inflammation-Diabetes Connection
Chronic inflammation is like a slow poison, gradually eroding the pancreas’s ability to function and paving the way for type 3c diabetes.
While it may start as a manageable condition, the long-term impact on insulin production and glucose regulation can be life-changing.
Understanding this link between chronic inflammation and type 3c diabetes is crucial for both prevention and treatment.
While this article does not offer solutions, it should serve as a wake-up call to the dangers of ignoring persistent inflammation.
References: