How Dominant Inheritance Impacts MODY Onset in Young Individuals?
- admin
- November 28, 2024
- 5:52 pm
- No Comments

Maturity-Onset Diabetes of the Young (MODY) is a unique form of diabetes caused by genetic mutations that affect insulin production and glucose regulation.
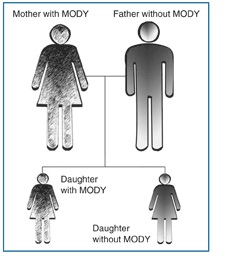
Unlike Type 1 and Type 2 diabetes, MODY follows an autosomal dominant inheritance pattern, making family history a critical factor in its diagnosis and progression.
Monogenic diabetes, particularly Maturity-Onset Diabetes of the Young (MODY), presents a unique insight into how genetics can influence the early onset of chronic conditions.
MODY, caused by mutations in a single gene, follows an autosomal dominant inheritance pattern, meaning an affected individual has a 50% chance of passing it on to their offspring.
In this article, BestDietarySuppplementforDiabetics will discuss how dominant inheritance contributes to MODY’s early onset in young individuals.
We will explore genetic mechanisms, the role of specific MODY-related genes, and real-life examples that illustrate its effects.
By the end, you will have a comprehensive understanding of the relationship between dominant inheritance and MODY in younger populations.
Index:
- What is MODY and its Genetic Basis?
- Understanding Dominant Inheritance
- Key Genes Involved in MODY Onset
- How Dominant Inheritance Impacts Early MODY Diagnosis
- Family History and Predictive Risk
- Case Study: Emily’s MODY Journey
- Broader Implications of Dominant Inheritance in MODY
- FAQs on How Dominant Inheritance Leads to MODY
- Conclusion
What is MODY and its Genetic Basis?
MODY is a rare, monogenic form of diabetes, often diagnosed in individuals under the age of 25.
Unlike Type 1 or Type 2 diabetes, MODY results from a single gene mutation affecting pancreatic beta-cell function and insulin secretion.
This monogenic nature makes MODY an ideal case study to understand the genetic foundation of chronic diseases.
The genes implicated in MODY follow an autosomal dominant inheritance pattern, meaning that just one defective copy of the gene from an affected parent is enough to cause the disease.
As a result, MODY typically appears in multiple generations within a family, affecting young individuals at a much higher rate compared to other diabetes types.
Dominant inheritance in MODY underscores how a single mutated allele is sufficient to trigger the condition.
This pattern of inheritance ensures predictable transmission within families, often leading to early detection in younger generations.
How Dominant Inheritance Leads to Early Onset?
- Reduced Genetic Buffering:
- In individuals with a dominant mutation, one functional allele cannot fully compensate for the defective gene.
- This limited genetic capacity accelerates the manifestation of MODY symptoms, such as glucose intolerance and insulin deficiency, during adolescence or early adulthood.
- Intergenerational Consistency:
- A parent with MODY has a 50% chance of passing the mutation to their offspring.
- This consistent transmission ensures that the condition remains prevalent across generations, enabling patterns to be identified within family histories.
- Family Screening Opportunities:
- Dominant inheritance simplifies the process of identifying at-risk individuals.
- Family history becomes a critical diagnostic tool, prompting early testing and intervention for younger relatives.
A study published in Diabetologia highlights how understanding dominant inheritance allows clinicians to recognize MODY cases earlier.
Early diagnosis ensures tailored management strategies, which are especially critical for young individuals to prevent long-term complications.
This predictable inheritance pattern serves as a cornerstone in both research and clinical practice for MODY.
Key Genes Involved in MODY Onset
Three primary genes—HNF1A, GCK, and HNF4A—account for the majority of MODY cases, with their dominant mutations significantly influencing the disease’s early onset.
- HNF1A (MODY3):
- This gene is critical for regulating beta-cell function and insulin production.
- Mutations in HNF1A result in an early decline in insulin secretion, causing glucose intolerance in affected individuals.
- A study published in the Journal of Clinical Endocrinology & Metabolism reports that HNF1A mutations are responsible for 30–50% of all MODY cases, making it the most common subtype.
- GCK (MODY2):
- GCK plays a central role in glucose sensing within beta cells, ensuring the proper regulation of insulin release.
- Mutations in this gene lead to stable, mild hyperglycemia that typically manifests during childhood or adolescence and does not progress significantly over time.
- HNF4A (MODY1):
- This gene contributes to pancreatic beta-cell differentiation and insulin secretion.
- Mutations result in progressive glucose intolerance and early-onset diabetes, often with additional features such as large birth weight.
Each of these genetic mutations demonstrates the direct impact of dominant inheritance on beta-cell dysfunction, elucidating the mechanisms behind MODY’s early manifestation.
Their study provides insights into precise diagnostic and therapeutic approaches, further emphasizing the importance of understanding the genetic roots of MODY.
How Dominant Inheritance Impacts Early MODY Diagnosis
Symptoms of Early Onset?
Young individuals with MODY often present with mild fasting hyperglycemia, absent of insulin resistance or autoimmunity, which differentiates it from Type 1 and Type 2 diabetes.
Early symptoms include:
- Frequent urination.
- Mild but persistent high blood sugar levels.
- Family history of early-onset diabetes.
Role of Genetic Testing:
Dominant inheritance patterns make genetic testing a powerful tool in diagnosing MODY. By identifying single-gene mutations, healthcare providers can differentiate MODY from other diabetes types, ensuring proper treatment.
This highlights the importance of early genetic testing in families with a history of diabetes.
Family History and Predictive Risk
Here is how it impacts you:
Why Family History Matters?
MODY’s autosomal dominant inheritance pattern highlights the critical role of family history in identifying at-risk individuals.
When one parent has MODY, there is a 50% likelihood of passing the mutation to their children. This high probability emphasizes the need for family-based screening and genetic counseling.
Recognizing the pattern of early-onset diabetes in family members often provides the first clue to a MODY diagnosis, making it essential for healthcare providers to inquire about multigenerational medical histories.
Case Study: Emily’s MODY Journey
Emily, a 19-year-old college student, experienced mild fasting hyperglycemia during a routine health check.
Her mother, diagnosed with diabetes in her late 20s, managed her condition using oral medication without requiring insulin. Recognizing the family pattern, Emily’s doctor recommended genetic testing, which confirmed the presence of an HNF1A mutation, diagnosing her with MODY3.
With this confirmation, her treatment shifted to sulfonylureas, significantly improving her glycemic control without insulin dependency.
Emily’s experience highlights the power of family history in shaping early and accurate diagnoses.
Understanding the hereditary nature of MODY allows individuals like Emily to receive timely, targeted interventions, ultimately improving long-term health outcomes.
Genetic insights paired with family awareness provide a roadmap for proactive disease management.
Broader Implications of Dominant Inheritance in MODY
Let us take a closer look at this perspective:
Impact on Family Planning
Dominant inheritance raises questions for young individuals with MODY about passing the condition to their children.
Genetic counseling provides valuable insights into the likelihood of transmission and potential interventions.
Healthcare Strategies
Understanding dominant inheritance allows for the development of tailored healthcare strategies, including:
- Family-based risk assessments.
- Preventative care for at-risk individuals.
- Personalized treatment plans for young MODY patients.
Ethical Considerations
The ability to predict MODY through genetic testing also brings ethical challenges, including decisions around disclosure, insurance, and family planning.
FAQs on How Dominant Inheritance Leads to MODY
Q-1: Why does dominant inheritance make MODY show up in the teens or 20s instead of childhood for many families?
A-1: With one altered gene enough to raise risk, half of children of an affected parent inherit it. Onset often waits for a “stress test” of the β-cell—puberty. Growth hormone and sex steroids increase physiologic insulin resistance, exposing a built-in secretion defect. The result: a previously normal fasting glucose begins to drift up, post-meal spikes linger longer, and subtle symptoms (thirst, nighttime urination) finally appear.
Q-2: My parent developed diabetes at 35, but I’m 16 and already high—how can the same dominant variant present earlier in me?
A-2: Dominant doesn’t mean identical. Age at onset varies with modifier genes, intrauterine environment (e.g., maternal hyperglycemia), early-life growth patterns, sleep/stress, and medication exposures. If your β-cells start with less “reserve,” the same variant crosses the clinical threshold sooner—especially during puberty or rapid growth—while your parent’s onset waited for pregnancy, weight gain, or midlife stress.
Q-3: How does dominant inheritance increase the risk of mislabeling young people as Type 1 or Type 2?
A-3: Teens with MODY may be lean (unlike typical Type 2) yet antibody-negative and still making insulin years after diagnosis (unlike classic Type 1). Without a family lens, clinicians may default to Type 1 in lean youth or Type 2 in heavier youth. A telltale pattern is “diabetes every generation,” onset usually <35, negative islet autoantibodies, and preserved C-peptide—signals to consider a MODY gene test instead of a lifetime of the wrong therapy.
Q-4: If MODY is dominant, when should younger siblings start surveillance—and what actually changes with an early label?
A-4: Begin gentle screening around late childhood (e.g., fasting glucose/HbA1c yearly, with an oral glucose check if borderline or during puberty). A confirmed MODY subtype shifts care: some HNF-gene carriers respond best to very low-dose sulfonylureas; glucokinase (GCK) carriers usually don’t need glucose-lowering drugs outside pregnancy; HNF1B carriers merit kidney/electrolyte monitoring. The payoff is targeted therapy and fewer emergencies from misclassification.
Q-5: Can a child have a dominant MODY even if neither parent is diagnosed—and what does that mean for their kids later?
A-5: Yes. A de novo variant can arise in the child, or a parent may carry mosaic changes that standard testing missed. Once present, the variant follows dominant rules going forward: each of that child’s future offspring has ~50% risk. Knowing this early enables preconception counseling, pregnancy planning for gene-specific fetal effects, and timely testing of relatives who might otherwise be mislabeled.
Conclusion:
Dominant inheritance plays a pivotal role in the early onset of MODY, ensuring its consistent presence across generations.
By understanding the genetic basis of this unique form of diabetes, healthcare providers can diagnose and manage MODY more effectively in young individuals.
Real-life examples, such as Emily’s story, highlight the importance of genetic testing and family history in guiding diagnosis and treatment.
Through advancements in genetic research and family-based healthcare strategies, the impact of MODY on younger generations can be better understood and managed.
References: