How Glucokinase Enzyme Mutations Contribute to MODY Diabetes?
- admin
- December 3, 2024
- 9:54 am
- No Comments
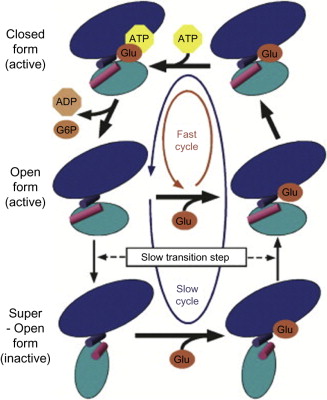
Maturity-Onset Diabetes of the Young (MODY) is a monogenic form of diabetes caused by mutations in genes critical for pancreatic beta-cell function.
Among the various forms of MODY, MODY2, caused by mutations in the glucokinase (GCK) gene, is one of the most prevalent.
Glucokinase plays a pivotal role in glucose sensing and regulation, making its mutation a key factor in the development of MODY diabetes.
This article by bestdietarysupplementfordiabetics.com explores how glucokinase enzyme mutations contribute to MODY diabetes, detailing the molecular mechanisms, clinical presentations, diagnostic techniques, and treatment implications.
Article Index:
- Introduction to Glucokinase and MODY2
- The Role of Glucokinase in Glucose Regulation
- Mechanisms of Glucokinase Mutations Leading to MODY2
- Clinical Features of MODY2
- Diagnosis of MODY2: Genetic Testing and Biomarkers
- Real-Life Example: Jane’s MODY2 Diagnosis
- Research Studies Supporting GCK-MODY Link
- Treatment and Management of MODY2
- FAQs on Glucokinase and MODY2
- Conclusion
Introduction to Glucokinase and MODY2
Glucokinase, often referred to as the “glucose sensor,” is an enzyme that catalyzes the phosphorylation of glucose to glucose-6-phosphate in pancreatic beta cells and liver hepatocytes.
This reaction is a critical first step in glucose metabolism, allowing beta cells to sense blood glucose levels accurately.
MODY2 arises when mutations in the GCK gene reduce or alter glucokinase activity, impairing glucose sensing and leading to mild, stable hyperglycemia.
Unlike other forms of diabetes, MODY2 typically does not involve insulin resistance or autoimmunity.
The Role of Glucokinase in Glucose Regulation
The primary function of glucokinase is to regulate insulin secretion in response to blood glucose levels.
Located in the pancreas, glucokinase determines the glucose threshold at which insulin is secreted.
By converting glucose to glucose-6-phosphate, it enables beta cells to “measure” glucose concentrations and trigger insulin release appropriately.
In the liver, glucokinase also facilitates glycogen synthesis and glucose utilization, playing a dual role in maintaining glucose homeostasis.
When glucokinase activity is impaired, the entire feedback loop between blood glucose levels and insulin secretion becomes disrupted.
Mechanisms of Glucokinase Mutations Leading to MODY2
A brief look at the mechanics:
A. Reduced Enzyme Activity:
Mutations in the GCK gene often result in reduced catalytic activity of glucokinase, increasing the glucose threshold required for insulin secretion. This leads to persistent hyperglycemia, as beta cells fail to respond to normal glucose levels.
B. Structural Abnormalities:
Some mutations alter the protein structure of glucokinase, reducing its stability and ability to bind glucose effectively. A study published in Diabetes (2019) demonstrated that structural changes in glucokinase reduce its glucose affinity, contributing directly to MODY2.
C. Altered Regulation:
Mutations may also affect the interaction of glucokinase with its regulatory proteins, such as glucokinase regulatory protein (GKRP) in the liver. This impacts glucose sensing and metabolism further, exacerbating hyperglycemia.
Clinical Features of MODY2
Patients with MODY2 display distinct characteristics that differentiate this condition from other forms of diabetes:
- Mild Fasting Hyperglycemia: Blood glucose levels typically range between 5.5–8 mmol/L, elevated yet significantly lower than those seen in uncontrolled Type 1 or Type 2 diabetes.
- Stable Glycemia: MODY2 exhibits a non-progressive nature, with glucose levels remaining relatively stable over time and rarely leading to severe complications.
- Family History: An autosomal dominant inheritance pattern is a hallmark of MODY2. Affected individuals often report multiple family members diagnosed with early-onset diabetes.
- No Insulin Resistance or Autoimmunity: Unlike Type 2 diabetes, MODY2 patients do not exhibit insulin resistance. Additionally, the absence of autoantibodies typically found in Type 1 diabetes underscores its unique etiology.
These clinical features ensure that MODY2 is easily distinguishable with accurate diagnostic tools and proper genetic evaluation.
Diagnosis of MODY2: Genetic Testing and Biomarkers
Here is how it all takes shape:
Here are the facts:
Genetic Testing:
Advanced techniques like Next-Generation Sequencing (NGS) and Polymerase Chain Reaction (PCR) are pivotal in identifying GCK mutations associated with MODY2. Genetic testing is particularly useful in cases with a strong family history of early-onset, non-insulin-dependent diabetes.
Biochemical Markers:
The clinical presentation of MODY2 often includes mild, persistent fasting hyperglycemia in the range of 5.5–8 mmol/L. Unlike other diabetes types, MODY2 lacks significant glycemic variability or markers of insulin resistance, making biochemical evaluation a key diagnostic tool.
Family Screening:
Screening at-risk family members is crucial. Identifying carriers of GCK mutations enables early management and prevents complications.
Supporting Evidence:
A study in Endocrine Reviews (2020) highlighted the role of genetic testing in improving diagnostic accuracy for MODY2. Early identification reduces misclassification, ensuring patients receive tailored management strategies rather than unnecessary interventions.
Jane’s MODY2 Diagnosis
Let me walk you through a classic case:
Background:
Jane, a 22-year-old college student, was surprised by consistently elevated fasting blood glucose levels of around 6.5 mmol/L during a routine check-up. She maintained a healthy lifestyle and was of normal weight, making her results unusual.
Evaluation:
Given her family history of early-onset diabetes, Jane’s endocrinologist recommended genetic testing. The results confirmed a mutation in the GCK gene, diagnosing her with MODY2, a form of diabetes caused by glucokinase enzyme mutations.
Management:
Unlike other types of diabetes, Jane’s condition required no pharmacological intervention. Her glucose levels remained stable and mild, and regular lifestyle monitoring was sufficient to manage her condition effectively.
Outcome:
Jane’s diagnosis led to genetic testing for her siblings. Another family member was identified with MODY2, allowing for early diagnosis and management. Jane’s case highlights the importance of genetic testing and family screening in identifying and managing MODY2.
Research Studies Supporting GCK-MODY Link
Numerous studies underscore the impact of GCK mutations in MODY2:
- Diabetologia (2021): Demonstrated that GCK mutations impair glucose sensing in beta cells, resulting in persistent yet mild hyperglycemia.
- Journal of Clinical Endocrinology & Metabolism (2018): Identified that GCK mutations contribute to approximately 30% of MODY cases within certain populations, highlighting their prevalence.
- Nature Genetics (2019): Explored how epigenetic modifications influence GCK mutation expression, offering deeper insights into clinical variability among MODY2 patients.
These findings solidify the role of GCK mutations in MODY2 and pave the way for improved diagnostic and therapeutic strategies.
Treatment and Management of MODY2
MODY2, caused by GCK mutations, requires a management approach distinct from other types of diabetes due to its unique clinical presentation.
- Minimal Pharmacological Intervention:
In most cases, medication is unnecessary. Lifestyle monitoring, such as maintaining a balanced diet and regular physical activity, paired with routine glucose monitoring, suffices to manage the mild hyperglycemia characteristic of MODY2. - Avoidance of Insulin Therapy:
Unlike Type 1 and Type 2 diabetes, insulin therapy is typically unnecessary for MODY2 patients. Insulin is only considered in rare situations where additional health conditions, such as pregnancy or illness, exacerbate hyperglycemia. - Family Counseling:
Genetic counseling is crucial in MODY2 management, given its autosomal dominant inheritance. Counseling educates families about the likelihood of inheritance, the importance of genetic testing, and the benefits of early diagnosis to prevent complications.
A study in Diabetes Care emphasizes that personalized care tailored to MODY2’s specific genetic and clinical features leads to better patient outcomes and reduces unnecessary medical interventions.
FAQs on Glucokinase and MODY2
Q-1: What does a glucokinase (GCK) mutation actually change inside a β-cell to cause MODY?
A-1: Glucokinase is the β-cell’s glucose “sensor.” When working normally, it kick-starts ATP production, closes KATP channels, depolarizes the membrane, and triggers calcium-dependent insulin release. Loss-of-function mutations raise the glucose threshold for that cascade. Insulin therefore turns on later—and less—for any given blood sugar. The result is lifelong, mild fasting hyperglycemia with small post-meal rises: the classic pattern of GCK-MODY (often called MODY2), which reflects a reset sensor rather than failing β-cells.
Q-2: Why do people with GCK-MODY often have stable sugars for years and few complications?
A-2: The core defect is calibration, not degeneration. Because the “on switch” for insulin is set higher, glucose runs modestly elevated but remains stable across life. First-phase insulin release is preserved; it just starts at a higher glucose. Without progressive β-cell loss, risks of microvascular complications are comparatively low when other risk factors are controlled. Many individuals don’t need glucose-lowering drugs except in specific circumstances like pregnancy.
Q-3: How does the liver’s version of glucokinase add to the MODY picture?
A-3: Hepatocytes use glucokinase to funnel glucose into glycogen and glycolysis after meals. Inactivating variants blunt this uptake and allow liver glucose output to persist longer. Combine that with β-cells that demand more glucose before releasing insulin, and you get the typical GCK-MODY profile: mildly high fasting glucose and a relatively small jump at two hours on an oral glucose test, rather than the steep rise seen when insulin secretion or action is broadly impaired.
Q-4: What clinical clues point to a GCK mutation rather than type 1 or type 2 diabetes?
A-4: Look for lifelong, stable mild fasting hyperglycemia often noticed incidentally; an autosomal-dominant pattern across generations; negative islet autoantibodies; preserved C-peptide; and a small 2-hour increment on an oral glucose tolerance test. People are frequently lean or average weight, and standard lifestyle measures keep numbers steady. Genetic confirmation prevents mislabeling as type 1 or type 2 and avoids unnecessary escalation to medications that won’t meaningfully shift the reset set-point.
Q-5: How does pregnancy change management for someone with GCK-MODY?
A-5: Care depends on whether the fetus inherits the variant. If the fetus has GCK-MODY, its pancreas “expects” the higher maternal glucose; aggressive treatment can risk growth restriction. If the fetus does not inherit the variant, maternal hyperglycemia can drive excess fetal growth, so tighter targets are warranted. Because fetal genotype isn’t known early, clinicians integrate growth scans and family genetics to guide therapy, aiming to protect both fetal growth and maternal safety.
Conclusion
Mutations in the glucokinase (GCK) enzyme play a pivotal role in MODY2 by impairing the beta cells’ ability to sense glucose levels accurately.
This disruption alters the threshold at which insulin is secreted, leading to mild but persistent fasting hyperglycemia.
Unlike other forms of diabetes, MODY2 is characterized by stable blood sugar levels, an absence of insulin resistance, and a strong genetic component, often affecting multiple family members.
Early diagnosis through advanced genetic testing, such as Next-Generation Sequencing (NGS), allows for precise identification of GCK mutations.
Clinical evaluations, including biomarkers and family history analysis, further refine the diagnostic process.
Proper diagnosis not only prevents misclassification with other diabetes types but also avoids unnecessary treatments, such as insulin therapy.
By understanding the molecular and clinical implications of GCK mutations, healthcare providers can develop tailored management strategies, ensuring optimal outcomes and a better quality of life for patients with MODY2.
References: