How HNF4A Mutations Impair Pancreatic Beta-Cell Development?
- admin
- January 1, 2025
- 11:47 am
- No Comments
The development of pancreatic beta cells is a highly regulated process that is crucial for maintaining glucose homeostasis in the human body.
Hepatocyte nuclear factor 4-alpha (HNF4A) is a transcription factor that plays a pivotal role in beta-cell differentiation and function.
Mutations in the HNF4A gene have been linked to maturity-onset diabetes of the young (MODY1) and neonatal diabetes, conditions characterized by insulin deficiency and glucose dysregulation.
In this article, bestdietarysupplementfordiabetics.com will delve into how HNF4A mutations disrupt beta-cell development, exploring the molecular mechanisms, genetic evidence, and clinical implications of these mutations.
Index:
- Introduction to HNF4A and Its Role in Beta-Cell Development
- Mechanisms by Which HNF4A Regulates Pancreatic Beta-Cell Development
- Impact of HNF4A Mutations on Beta-Cell Differentiation
- Beta-Cell Function and Insulin Secretion in HNF4A Mutations
- Real-Life Case Studies: Effects of HNF4A Mutations
- Clinical and Genetic Studies Linking HNF4A Mutations to Diabetes
- FAQs on HNF4A Impair & Pancreatic Beta-Cell Development
- Conclusion
Introduction to HNF4A and Its Role in Beta-Cell Development
HNF4A, a member of the nuclear receptor family, is a transcription factor critical for the proper development and function of pancreatic beta cells.
It regulates genes involved in glucose metabolism, insulin secretion, and cellular differentiation. During embryonic development, HNF4A ensures that progenitor cells in the pancreas mature into functional beta cells capable of producing insulin.
Mutations in HNF4A disrupt these developmental processes, leading to structural and functional beta-cell abnormalities.
These mutations are often inherited in an autosomal dominant manner and are implicated in various forms of monogenic diabetes.
Understanding the mechanisms of HNF4A mutations is key to developing targeted therapies for these conditions.
Mechanisms by Which HNF4A Regulates Pancreatic Beta-Cell Development
A quick look at the facts:
Role in Pancreatic Progenitor Cell Differentiation:
HNF4A is expressed early in pancreatic progenitor cells, where it regulates key transcription factors such as PDX1, NEUROG3, and NKX6.1.
These factors drive the differentiation of progenitor cells into endocrine cells, including beta cells.
Mutations in HNF4A can impair the expression of these downstream genes, stalling the maturation of functional beta cells.
Regulation of Insulin Gene Transcription:
HNF4A directly influences insulin gene expression by interacting with other transcription factors like HNF1A and PDX1.
Disruptions in HNF4A activity result in reduced insulin transcription and secretion, contributing to hyperglycemia.
Maintaining Beta-Cell Identity:
HNF4A helps maintain the identity of beta cells by regulating genes involved in metabolic processes and cellular integrity.
Mutations in HNF4A can lead to dedifferentiation, where beta cells lose their functional characteristics and adopt a less specialized state.
Scientific Evidence:
A study by Gupta et al. (2015) in Cell Metabolism demonstrated that HNF4A knockout in mice led to impaired beta-cell differentiation and reduced insulin secretion, confirming its essential role in beta-cell development.
Impact of HNF4A Mutations on Beta-Cell Differentiation
Loss-of-Function Mutations:
Loss-of-function mutations in the HNF4A gene compromise its ability to activate key target genes essential for beta-cell development and function.
During fetal development, HNF4A plays a pivotal role in the differentiation of pancreatic progenitor cells into mature beta cells.
Mutations in this gene disrupt this process, leading to a reduced beta-cell mass, a defining characteristic of neonatal diabetes.
A diminished beta-cell population directly impacts insulin production and secretion, laying the groundwork for persistent hyperglycemia in affected individuals.
Disrupted Mitochondrial Function:
HNF4A also regulates genes critical for mitochondrial function, which is central to energy production in beta cells.
Mutations in HNF4A impair mitochondrial ATP production, reducing the energy required for insulin granule exocytosis.
This dual impact—on beta-cell mass and ATP-driven insulin secretion—creates a compounding effect, significantly worsening glucose intolerance in individuals with HNF4A mutations.
Real-Life Example:
In a family affected by MODY1, genetic analysis identified a point mutation in the HNF4A gene.
Members of the family displayed reduced beta-cell mass and impaired insulin secretion, even at fasting glucose levels.
This real-world scenario underscores the profound impact of HNF4A mutations on beta-cell development and highlights the importance of genetic analysis in diagnosing and understanding monogenic diabetes.
Beta-Cell Function and Insulin Secretion in HNF4A Mutations
A quick look at these facts:
Defective Insulin Secretion Pathway:
Mutations in the HNF4A gene disrupt the finely tuned process of insulin secretion by impairing glucose-stimulated insulin release.
This dysfunction stems from reduced ATP production, which hinders the closure of ATP-sensitive potassium (KATP) channels.
As a result, the necessary membrane depolarization and subsequent calcium influx required for insulin granule exocytosis are diminished.
Additionally, the expression of KATP channel subunits, integral to beta-cell functionality, is altered, further compromising insulin secretion.
Reduced Beta-Cell Responsivenes:
Beta cells carrying HNF4A mutations exhibit a reduced response to incretins, such as GLP-1, which normally enhance insulin release after meals.
This diminished responsiveness exacerbates postprandial hyperglycemia, adding complexity to glucose regulation.
Scientific Evidence:
A study by Pearson et al. (2004) in Nature Genetics highlighted that individuals with HNF4A mutations had significantly lower insulinogenic indices.
This finding underscores the impaired ability of beta cells to respond adequately to glucose stimuli, marking a key deficit in patients with HNF4A-related diabetes.
Emma’s Neonatal Diabetes Due to HNF4A Mutation
Emma, a six-month-old infant, presented with persistent hyperglycemia and poor weight gain. Genetic testing revealed a loss-of-function mutation in the HNF4A gene, which impaired her pancreatic beta-cell development and insulin secretion.
To stabilize her blood glucose levels, insulin therapy was initiated.
As Emma grew, she also exhibited mild hepatic dysfunction, a known extra-pancreatic feature of HNF4A-related diabetes, underscoring the broader metabolic implications of this genetic mutation.
This case highlights the importance of early genetic diagnosis for managing neonatal diabetes and monitoring associated complications.
A Family with MODY1
In a family spanning three generations, a missense mutation in HNF4A was identified as the cause of maturity-onset diabetes of the young (MODY1). The grandfather, diagnosed in his 50s, required lifelong insulin therapy.
His son managed his diabetes with diet modifications alone, reflecting a milder phenotype. The granddaughter, diagnosed during adolescence, displayed only mild fasting hyperglycemia.
This case underscores the variable expressivity of HNF4A mutations, influenced by genetic and environmental factors. It also emphasizes the importance of genetic counseling for early diagnosis, personalized treatment, and preventive care for at-risk family members.
Early interventions tailored to individual needs can significantly improve clinical outcomes and quality of life.
Clinical and Genetic Studies Linking HNF4A Mutations to Diabetes
Yamagata et al., 1996 (Nature)
This foundational study identified HNF4A as a master regulator of beta-cell function. It linked mutations in HNF4A to maturity-onset diabetes of the young (MODY1), establishing the gene’s critical role in glucose homeostasis.
Stanley et al., 2016 (The Journal of Clinical Endocrinology & Metabolism)
This research emphasized the clinical variability of HNF4A mutations. It highlighted cases ranging from neonatal diabetes with severe beta-cell dysfunction to mild adult-onset diabetes, underlining the broad phenotypic spectrum associated with these mutations.
Gupta et al., 2015 (Cell Metabolism)
This study demonstrated the essential role of HNF4A in beta-cell differentiation using animal models. It further explored how disrupted HNF4A function leads to impaired glucose metabolism and reduced insulin secretion.
Implications for Genetic Testing
The discovery of HNF4A mutations has transformed diabetes diagnosis and management.
Early genetic testing enables personalized treatment strategies, such as sulfonylurea therapy in place of insulin for some cases.
Accurate genetic diagnosis not only improves clinical outcomes but also allows for better counseling and monitoring of affected families.
FAQs on HNF4A Impair & Pancreatic Beta-Cell Development
Q-1: How do HNF4A mutations affect pancreatic beta-cell development?
A-1: HNF4A mutations disrupt the normal function of the HNF-4α protein, a key transcription factor in pancreatic beta-cells. This disruption impairs the expression of genes essential for beta-cell differentiation and insulin secretion, leading to beta-cell dysfunction and reduced insulin production.
Q-2: What are the specific genetic targets influenced by HNF4A in beta-cells?
A-2: HNF4A regulates several genes critical for beta-cell function, including those involved in glucose metabolism and insulin secretion. Mutations in HNF4A can alter the expression of these target genes, compromising beta-cell development and function.
Q-3: How do HNF4A mutations contribute to the development of maturity-onset diabetes of the young (MODY1)?
A-3: In MODY1, HNF4A mutations lead to impaired beta-cell development and function, resulting in reduced insulin secretion. This insulin deficiency causes elevated blood glucose levels, characteristic of MODY1.
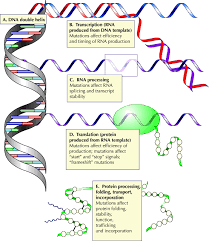
Q-4: Are there different types of mutations in HNF4A, and how do they affect beta-cell development?
A-4: HNF4A mutations include missense, nonsense, and frameshift mutations, each affecting the protein’s function differently. These mutations can disrupt the transcriptional regulation of genes essential for beta-cell development, leading to varying degrees of beta-cell dysfunction.
Q-5: How does the P2 promoter of HNF4A influence beta-cell development?
A-5: The P2 promoter of HNF4A is crucial for the expression of specific HNF-4α isoforms in beta-cells. Mutations in this promoter region can impair the production of these isoforms, disrupting beta-cell differentiation and function.
Q-6: Can HNF4A mutations affect insulin secretion in addition to beta-cell development?
A-6: Yes, HNF4A mutations not only impair beta-cell development but also affect insulin secretion. The altered expression of genes involved in insulin production and secretion leads to reduced insulin levels, contributing to hyperglycemia.
Conclusion
HNF4A mutations significantly impair pancreatic beta-cell development by disrupting transcriptional networks, metabolic pathways, and insulin secretion mechanisms.
These mutations lead to a spectrum of conditions, from neonatal diabetes to MODY1, characterized by varying degrees of insulin deficiency and glucose dysregulation.
Continued research into the molecular mechanisms of HNF4A mutations will enhance our ability to diagnose and treat these conditions, offering hope for improved outcomes.
Scientific advancements in understanding HNF4A’s role in beta-cell biology underscore the importance of integrating genetic testing into clinical practice.
By addressing the root cause of beta-cell dysfunction, we can pave the way for targeted therapies that improve the lives of individuals with HNF4A-related diabetes.
References: