How Insulin Gene Mutations Disrupt Proinsulin Folding?
- admin
- December 15, 2024
- 6:24 pm
- No Comments
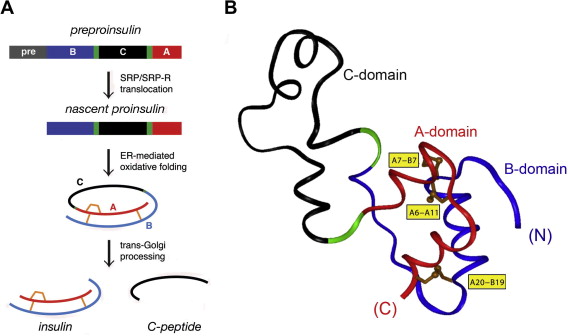
Proinsulin folding is a critical step in the production of insulin, a hormone essential for glucose regulation.
The insulin (INS) gene encodes proinsulin, a precursor molecule that undergoes complex folding processes in the endoplasmic reticulum (ER) of pancreatic beta cells to form active insulin.
Mutations in the INS gene disrupt this process, leading to misfolded proinsulin and impaired insulin production.
This article by bestdietarysupplementfordiabetics.com delves into how and why insulin gene mutations hinder proinsulin folding, exploring the molecular mechanisms, physiological consequences, and real-world implications for affected individuals.
We will discuss:
- The role of proinsulin in insulin synthesis
- Mechanisms of proinsulin folding
- The impact of INS mutations on folding and beta-cell function
- Case studies illustrating the clinical significance
- Research studies highlighting the connection
Table of Contents:
- The Role of Proinsulin in Insulin Synthesis
- Mechanisms of Proinsulin Folding
- Importance of Disulfide Bonds
- Molecular Chaperones in Folding
- INS Gene Mutations and Their Effects
- Missense Mutations
- Frameshift Mutations
- Dominant-Negative Effects
- Real-Life Case Studies
- Case Study 1: Neonatal Diabetes
- Case Study 2: Familial Hyperinsulinemia
- FAQs on Insulin Gene Mutation & Proinsulin Folding
- Scientific Evidence Linking INS Mutations to Proinsulin Misfolding
The Role of Proinsulin in Insulin Synthesis
Insulin is synthesized as proinsulin, a single-chain protein consisting of the A-chain, B-chain, and connecting C-peptide.
This precursor undergoes folding and enzymatic cleavage to form mature insulin, a two-chain molecule responsible for glucose uptake.
Proper proinsulin folding ensures that the A- and B-chains align correctly for disulfide bond formation, stabilizing the molecule.
Any disruption in this process compromises insulin’s biological function, resulting in glucose dysregulation.
Mechanisms of Proinsulin Folding
Here is how this unfolds:
Importance of Disulfide Bonds:
Proinsulin folding involves the formation of three critical disulfide bonds: two between the A- and B-chains and one within the A-chain.
These bonds stabilize the insulin structure, enabling it to function correctly.
Mutations in the INS gene can interfere with disulfide bond formation, leading to misfolded or partially folded proinsulin.
For example, the R89C mutation substitutes arginine with cysteine, introducing an additional disulfide bond that destabilizes the structure.
This prevents proinsulin from adopting its native conformation, leading to ER stress and beta-cell dysfunction.
Molecular Chaperones in Folding:
Molecular chaperones, such as BiP and GRP94, assist in proinsulin folding by preventing aggregation and facilitating proper disulfide bond formation.
When INS mutations produce unstable proinsulin variants, these chaperones become overwhelmed, triggering the unfolded protein response (UPR).
Prolonged UPR activation can induce beta-cell apoptosis, further reducing insulin production.
INS Gene Mutations and Their Effects
Let us walk you through these in brief:
Missense Mutations:
Missense mutations involve single nucleotide changes that result in amino acid substitutions.
For instance, the C96Y mutation disrupts the A-chain’s intramolecular disulfide bond, leading to misfolded proinsulin.
A study in Human Molecular Genetics (Edghill et al., 2008) demonstrated that such mutations are a common cause of permanent neonatal diabetes mellitus (PNDM).
Frameshift Mutations:
Frameshift mutations occur due to nucleotide insertions or deletions, altering the reading frame of the INS gene.
These mutations produce truncated or abnormal proinsulin proteins that are nonfunctional.
For example, the G32fsdelC mutation generates a truncated proinsulin variant that accumulates in the ER, causing cellular stress and beta-cell apoptosis.
Dominant-Negative Effects:
Some INS mutations exhibit dominant-negative effects, where mutant proinsulin interferes with the folding or secretion of normal proinsulin.
This mechanism is particularly damaging, as even a single defective allele can significantly impair insulin production.
A study in Diabetes (Colombo et al., 2010) highlighted the impact of dominant-negative mutations in exacerbating beta-cell dysfunction.
Real-Life Cases Highlighting the Impact of INS Gene Mutations
A quick look at how this takes shape in the form of two examples:
Neonatal Diabetes in Infant Emma:
Emma, a three-month-old infant, presented with severe hyperglycemia, lethargy, and failure to thrive, prompting her pediatrician to order genetic testing.
The results revealed a missense mutation (R89H) in the INS gene, disrupting the formation of disulfide bonds essential for proper proinsulin folding.
This defect caused a buildup of misfolded proinsulin in the endoplasmic reticulum (ER), triggering ER stress and impairing beta-cell function.
With an early diagnosis, Emma transitioned from insulin injections to oral sulfonylureas, which partially restored her beta-cell function by enhancing insulin secretion.
Within weeks, her glucose levels stabilized, and her growth trajectory improved.
Emma’s case emphasizes the critical role of genetic testing in neonatal diabetes, as targeted interventions can significantly enhance management and outcomes.
Familial Hyperinsulinemia in Siblings:
In a family with a history of diabetes, two siblings displayed high fasting insulin levels despite normal blood glucose, leading to symptoms such as weight gain and fatigue.
Genetic analysis identified a frameshift mutation (G32fsdelC) in the INS gene.
The mutation produced a defective proinsulin protein that interfered with the secretion of normal insulin, demonstrating a dominant-negative effect.
The siblings underwent lifestyle interventions, including dietary modifications focused on low-glycemic foods and a structured exercise program.
These measures helped regulate their insulin levels and improved their metabolic profiles.
This case highlights the importance of recognizing familial patterns of INS mutations and the need for tailored interventions to mitigate the effects of proinsulin misfolding.
Groundbreaking Research on INS Mutations and Proinsulin Misfolding
Edghill et al., 2008 (Human Molecular Genetics)
This pivotal study established missense mutations in the INS gene as a primary cause of permanent neonatal diabetes mellitus (PNDM).
The researchers detailed how specific amino acid substitutions, such as R89H, disrupt disulfide bond formation, preventing proinsulin from achieving its correct three-dimensional structure.
This misfolding leads to ER stress and beta-cell dysfunction, underlining the molecular roots of this condition.
Colombo et al., 2010 (Diabetes)
The study focused on dominant-negative mutations and their impact on insulin production.
It demonstrated that mutant proinsulin proteins interfere with the folding and secretion of normal insulin, compounding beta-cell stress.
This research emphasized the heightened risk of insulin deficiency when even one INS allele is mutated, offering new insights into the genetic mechanisms of diabetes.
Støy et al., 2007 (Diabetologia)
This landmark research linked INS mutations to disrupted proinsulin folding and highlighted their clinical significance in neonatal diabetes.
By examining affected families, the study identified mutations like G32fsdelC as significant contributors to severe beta-cell dysfunction, emphasizing the importance of early diagnosis.
Molven et al., 2008 (Human Mutation)
Focusing on frameshift mutations, this study revealed their role in triggering ER stress and beta-cell apoptosis. Mutant proinsulin produced through frameshift errors was shown to be toxic to beta cells, reducing insulin output.
These findings paved the way for potential therapeutic approaches targeting ER stress alleviation to preserve beta-cell function.
These studies collectively provide a comprehensive understanding of how INS gene mutations impair proinsulin folding and disrupt insulin production.
FAQs on Insulin Gene Mutation & Proinsulin Folding
Q-1: What exactly goes wrong in proinsulin folding when the insulin (INS) gene is mutated?
A-1: Proinsulin must form three precise disulfide bonds as it folds in the endoplasmic reticulum. Many INS mutations destabilize this choreography, leading to mispaired bonds or a partially unfolded scaffold. The misfolded molecules get stuck in quality-control checkpoints instead of moving to secretory granules. As they accumulate, the cell’s stress sensors switch on, lowering the fraction of correctly folded proinsulin that becomes mature insulin.
Q-2: Why can a single mutated INS allele cause diabetes even when the other allele is normal?
A-2: Certain mutant proinsulin molecules interact with normal (wild-type) proinsulin while both are folding. These “bad partners” stick together and trap the normal protein in the endoplasmic reticulum, a dominant-negative effect. Because fewer correctly folded molecules reach the granules, overall insulin output falls. The problem isn’t just having less normal gene—it’s the mutant protein actively blocking its healthy counterpart.
Q-3: How early does misfolding show up, and how does it damage beta cells over time?
A-3: Misfolding is detectable before blood sugars rise, as retained proinsulin forms higher-order complexes in the endoplasmic reticulum. Chronic build-up triggers the unfolded protein response: translational slowdowns, upregulated chaperones, and, if stress persists, pathways that impair cell function and survival. Over months to years, this proteotoxic pressure shrinks the pool of effective insulin-secreting beta cells, converting a folding defect into clinically significant insulin deficiency.
Q-4: Are some mutation types more likely to derail folding than others?
A-4: Yes. Changes that alter cysteine residues tend to be especially disruptive because they directly rewire the disulfide map. But non-cysteine substitutions can also destabilize the core structure, slow oxidative folding, or increase aberrant self-association—all of which raise the odds of endoplasmic-reticulum retention. Different sequence changes converge on the same outcome: more misfolded proinsulin, fewer export-ready molecules.
Q-5: How do researchers know the primary problem is folding, not a downstream secretion glitch?
A-5: Several experimental clues point to folding as the bottleneck. When cell redox conditions are adjusted to favor native bond formation, some mutants produce more export-competent proinsulin, indicating that improving folding boosts secretion. Increasing overall protein load or cellular stress makes retention worse for both mutant and wild-type molecules, consistent with a capacity issue in the folding environment. These patterns align with a folding-centric mechanism driving the insulin shortfall.
Conclusion
Mutations in the INS gene disrupt proinsulin folding through mechanisms such as impaired disulfide bond formation, ER stress, and dominant-negative effects.
These disruptions compromise insulin production, leading to conditions like neonatal diabetes and familial hyperinsulinemia.
Real-life cases and scientific evidence underscore the importance of early genetic diagnosis and targeted interventions in managing these conditions.
Understanding the intricate relationship between INS mutations and proinsulin folding is crucial for developing future therapies aimed at preserving beta-cell function.
References: