How Mutations in the HNF1A Gene Lead to Impaired Insulin Secretion?
- admin
- November 27, 2024
- 10:30 am
- No Comments
Insulin secretion is a cornerstone of glucose regulation, and its dysfunction is often linked to genetic factors.
Among these, mutations in the HNF1A gene are particularly significant.
This article explores how HNF1A mutations impair insulin secretion, the biochemical pathways involved, and the broader implications for metabolic health.
BestDietarySupplementforDiabetics research staff shall examine real-life examples, scientific studies, and the critical role of the HNF1A transcription factor in glucose metabolism.
Article Index:
- What is the HNF1A Gene?
- Role of HNF1A in Insulin Secretion
- What is the HNF1A-MODY Gene and Its Function?
- Mechanisms of Impaired Insulin Secretion Due to HNF1A Mutations
- Real-Life Example: Living with HNF1A-MODY
- FAQs on Insulin Secretion & HNF1A Mutation
- Broader Implications: Diseases Linked to HNF1A Mutations
- Conclusion
What is the HNF1A Gene?
The HNF1A gene encodes hepatocyte nuclear factor-1 alpha (HNF1α), a transcription factor crucial for regulating metabolic processes.
Primarily expressed in the liver, pancreas, and kidneys, HNF1A plays a key role in maintaining glucose homeostasis.
What is the function of the HNF1A transcription factor?
It governs the expression of genes essential for glucose metabolism, insulin biosynthesis, and the survival of pancreatic beta cells.
In the pancreas, HNF1A ensures the efficient production and release of insulin by regulating glucose transporters like GLUT2, which facilitates glucose entry into beta cells.
In the liver, it controls genes involved in glycogen storage and glucose release, maintaining balanced blood sugar levels.
Its activity in the kidneys helps manage glucose reabsorption and excretion.
When mutations occur in the HNF1A gene, the transcription factor’s regulatory capacity diminishes.
This leads to disrupted insulin production, impaired glucose sensing, and increased blood sugar levels—hallmarks of Maturity-Onset Diabetes of the Young (MODY).
Understanding what is the HNF1A gene mutation provides critical insight into its metabolic consequences.
A study published in Diabetes Care highlights that individuals with HNF1A mutations often exhibit early-onset diabetes with unique characteristics, making this gene a focal point in diabetes research and personalized treatment approaches.
Role of HNF1A in Insulin Secretion
The HNF1A transcription factor is indispensable for maintaining the functionality of insulin-producing beta cells in the pancreas.
What is the function of HNF1A transcription factor?
It regulates critical genes involved in glucose metabolism, insulin biosynthesis, and beta-cell maintenance.
This includes controlling the production of enzymes and transporters essential for glucose sensing and insulin secretion.
One of the key targets of HNF1A is the GLUT2 glucose transporter, which facilitates glucose entry into beta cells.
GLUT2 is pivotal for glucose sensing, a process that triggers insulin release when blood sugar levels rise.
Mutations in HNF1A disrupt its ability to upregulate GLUT2 expression, impairing glucose-stimulated insulin secretion.
This deficiency results in elevated blood glucose levels and early-onset diabetes, characteristic of MODY3.
Furthermore, HNF1A supports beta-cell survival by regulating genes involved in cellular stress responses and metabolic balance.
Without its proper function, beta cells become more susceptible to dysfunction and apoptosis (cell death), compounding the insulin secretion defect.
A study in Nature Genetics highlights the cascading effect of HNF1A mutations, noting that they not only hinder insulin production but also exacerbate glucose dysregulation over time.
These findings emphasize the transcription factor’s critical role in maintaining pancreatic health and effective glucose regulation.
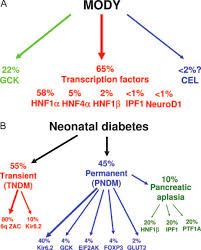
What is the HNF1A-MODY Gene and Its Function?
The HNF1A-MODY gene mutation is a defining cause of MODY3, the most prevalent form of Maturity-Onset Diabetes of the Young (MODY).
Unlike the multifactorial origins of Type 1 and Type 2 diabetes, MODY3 arises from a single gene mutation that disrupts the function of the HNF1A transcription factor.
This factor plays a pivotal role in regulating genes essential for glucose metabolism and insulin secretion in pancreatic beta cells.
MODY3 typically manifests with mild fasting hyperglycemia and an early onset, often before age 25.
Patients often respond well to sulfonylureas rather than insulin, offering a unique treatment pathway.
Recognizing what are the symptoms of HNF1A-MODY—such as increased sensitivity to oral medications and stable blood sugar levels with targeted therapy—is crucial for distinguishing it from other diabetes forms.
Understanding this gene’s role and symptoms aids in precise diagnosis and effective management strategies.
Mechanisms of Impaired Insulin Secretion Due to HNF1A Mutations
a) Disrupted Transcriptional Control
What is the function of HNF1 alpha?
It orchestrates the transcription of critical beta-cell genes. Mutations lead to insufficient production of insulin and glucose transporters, such as GLUT2.
A study in the Journal of Clinical Investigation found that HNF1A mutations significantly reduced beta-cell functionality in patients with MODY3.
b) Beta-Cell Dysfunction
Mutations cause structural changes in the beta cells, impairing their ability to respond to glucose.
This explains what is the gene mutation causing insulin resistance and its downstream effects on beta-cell sensitivity.
c) Epigenetic Factors
Environmental and epigenetic modifications exacerbate the effects of HNF1A mutations.
Poor diet or stress can worsen beta-cell function, illustrating what if a mutation occurred in the human insulin gene and how external factors amplify the genetic defect.
d) Altered Protein Synthesis
A hypothetical question often posed is, “What if a mutation occurred in the human insulin gene and the first triplet was changed to CCG?”
Such a mutation would lead to altered protein folding, rendering insulin ineffective.
Similarly, HNF1A mutations can affect proteins downstream in the insulin secretion pathway.
Living with HNF1A-MODY
Anna, a 24-year-old medical student, began experiencing persistent mild hyperglycemia during routine health checks.
Initially misdiagnosed with Type 1 diabetes, her condition didn’t fully align with the symptoms. Intrigued, her doctor explored genetic testing, which confirmed a mutation in Anna’s HNF1A gene, leading to a diagnosis of MODY3.
MODY3, caused by HNF1A mutations, is distinct from traditional diabetes forms.
While many ask, “What is the insulin gene mutation?” MODY3 arises from mutations that impair the function of the HNF1A transcription factor, critical for insulin production and secretion.
Anna’s treatment shifted from insulin to sulfonylureas, oral medications that enhanced her beta-cell function effectively.
Her blood sugar levels stabilized without the need for insulin injections, dramatically improving her quality of life.
Anna’s story emphasizes the importance of accurate diagnosis and personalized care in managing conditions tied to genetic mutations like MODY3, showcasing how precision medicine transforms outcomes.
FAQs on Insulin Secretion & HNF1A Mutations
Q-1: What’s the first step in insulin release that HNF1A disrupts?
A-1: Glucose sensing. HNF1A is a transcription controller for β-cell “entry” and processing machinery (e.g., GLUT2/SLC2A2, key glycolytic enzymes). When its signal is weak, glucose gets under-sensed and under-processed, so ATP rises less after a meal. With a smaller ATP surge, KATP channels don’t close robustly, electrical depolarization is blunted, and the all-important first-phase insulin burst arrives late and weak.
Q-2: Why do people with HNF1A variants spill urine glucose at relatively lower blood sugar?
A-2: Beyond the pancreas, HNF1A helps set kidney transport capacity. Reduced HNF1A activity lowers expression of glucose reuptake transporters in the proximal tubule, dropping the renal threshold for glycosuria. Clinically, that means positive urine glucose even with only mild hyperglycemia—and frequent thirst/urination out of proportion to meter readings.
Q-3: How does HNF1A failure change the β-cell’s “fuel → electricity → insulin” coupling?
A-3: Three links weaken: (1) Fueling: fewer transcripts for enzymes that turn glucose into ATP; (2) Excitation: a smaller ATP/ADP rise leaves KATP channels more open, limiting calcium entry; (3) Exocytosis: HNF1A also influences genes for granule docking/priming, so even when calcium arrives, fewer granules are release-ready. The net effect is low amplifying and triggering pathways, especially after carbohydrate-heavy meals.
Q-4: Why do tiny doses of sulfonylureas work unusually well in HNF1A-MODY?
A-4: Sulfonylureas bypass the faulty sensing step by directly closing KATP channels. That substitutes for the missing ATP signal and restores membrane depolarization and calcium influx, reviving insulin pulses from otherwise intact granules. Because the downstream machinery still works, small doses go a long way—making education on hypoglycemia prevention crucial.
Q-5: Are there non-pancreatic “clues” that point to HNF1A-driven secretion defects?
A-5: Yes—use the multi-organ fingerprint. Typical clues include: (a) Low hs-CRP for the degree of hyperglycemia (HNF1A regulates hepatic CRP production); (b) Lower glycosuria threshold (renal signature above); (c) Preserved C-peptide years after diagnosis (distinguishes from autoimmune loss); (d) Autosomal-dominant family pattern with early-onset diabetes in lean relatives. Together with a strong response to low-dose sulfonylureas, these features point to a primary insulin-secretion problem rooted in HNF1A rather than classic insulin resistance.
Broader Implications: Diseases Linked to HNF1A Mutations
Here are the ailments that might strike you at will:
a) MODY3 and Related Conditions
What are 3 diseases caused by mutations in HNF1A?
The primary conditions include:
b) MODY3 (Diabetes):
Characterized by impaired insulin secretion and glucose regulation, often requiring early intervention.
- Hepatic Adenomas: These benign liver tumors result from altered gene expression in hepatocytes, highlighting HNF1A’s role in liver function.
- Kidney Dysfunction: Reduced filtration and other renal impairments occur due to HNF1A’s regulatory role in kidney gene expression.
c) Systemic Impact:
HNF1A mutations have far-reaching effects beyond the pancreas.
What are hepatocyte nuclear factors?
These are transcription factors, including HNF1A, that regulate critical metabolic processes in the liver and kidneys.
Mutations disrupt cellular activities, leading to widespread metabolic issues, including heightened risks for metabolic syndrome and cardiovascular diseases, underscoring the systemic importance of HNF1A.
Takeaway:
Mutations in the HNF1A gene profoundly impact insulin secretion by disrupting beta-cell function, glucose transporter expression, and transcriptional regulation.
These genetic defects not only cause MODY3 but also predispose individuals to broader metabolic and systemic complications.
Research into how osteopathy helps posture correction is akin to the tailored strategies required for HNF1A mutations—both address underlying dysfunction to restore balance.
Understanding how often should you have osteopathy or how frequently to monitor blood glucose levels in MODY3 patients reflects the need for personalized, ongoing care.
By unraveling the molecular mechanisms of HNF1A mutations, we can improve diagnostic precision and treatment outcomes for individuals affected by this condition.
References: