How Mutations in the INS Gene Impair Insulin Production in Neonates?
- admin
- December 12, 2024
- 12:24 pm
- No Comments
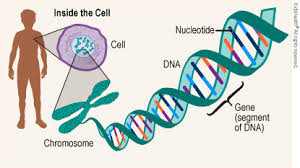
Neonatal diabetes mellitus (NDM) is a rare condition characterized by severe hyperglycemia in the first six months of life.
Unlike other forms of diabetes, NDM is often rooted in genetic anomalies, particularly mutations in the INS gene, which encodes insulin, a critical hormone for glucose regulation.
These mutations disrupt the synthesis, folding, or secretion of insulin, resulting in insufficient glucose metabolism in neonates.
This article explores how mutations in the INS gene impair insulin production, diving into the molecular mechanisms, genetic implications, and real-life cases.
Backed by scientific research, BestDietarySupplementforDiabetics provides a comprehensive analysis of this condition, focusing solely on the role of INS mutations.
Table of Contents:
- Introduction to the INS Gene and Neonatal Diabetes
- The Molecular Role of the INS Gene in Insulin Production
- 2.1. Insulin Synthesis and Folding
- 2.2. Role of Proinsulin Processing in Beta Cells
- How Mutations in the INS Gene Impair Insulin Production
- 3.1. Missense Mutations and Protein Misfolding
- 3.2. Frameshift Mutations and Truncated Insulin
- 3.3. Dominant-Negative Mutations and Beta-Cell Stress
- Real-Life Case Studies
- 4.1. Case Study: Missense Mutation in a Newborn
- 4.2. Case Study: Familial INS Gene Mutations
- FAQs on INS Gene and Neonatal Diabetes
- Conclusion
Introduction to the INS Gene and Neonatal Diabetes
The INS gene, situated on chromosome 11, plays a pivotal role in the body’s ability to regulate blood glucose levels.
It encodes proinsulin, the precursor molecule that undergoes intricate folding and enzymatic processing within pancreatic beta cells to produce active insulin.
Insulin is essential for facilitating glucose uptake into cells, ensuring energy production, and maintaining metabolic balance.
Mutations in the INS gene disrupt these finely tuned processes, impairing insulin production, folding, or secretion.
These disruptions lead to permanent neonatal diabetes mellitus (PNDM), a rare but severe condition that manifests within the first six months of life and requires lifelong management.
Mutated proinsulin proteins often misfold or fail to process correctly, leading to endoplasmic reticulum (ER) stress, beta-cell dysfunction, and chronic hyperglycemia.
Groundbreaking studies, such as one published in Diabetologia (Støy et al., 2007), identify INS gene mutations as one of the most common genetic causes of PNDM.
These findings emphasize the gene’s central role in early-onset diabetes.
This article explores the molecular mechanisms by which INS gene mutations impair insulin production, delving into the cascading effects on neonatal health and real-world examples of their impact on affected individuals and families.
Understanding these mechanisms provides critical insights into managing and diagnosing neonatal diabetes effectively.
The Molecular Role of the INS Gene in Insulin Production
Here is how it all takes shape:
Insulin Synthesis and Folding:
The INS gene encodes proinsulin, a precursor to functional insulin.
Proinsulin undergoes a meticulously regulated folding process within the endoplasmic reticulum (ER) of pancreatic beta cells.
This folding involves the formation of disulfide bonds, which stabilize the three-dimensional structure of the insulin molecule.
These bonds are crucial for ensuring that insulin achieves its active conformation, necessary for its biological function in glucose regulation.
However, mutations in the INS gene can disrupt this delicate process.
For instance, altered amino acid sequences due to missense mutations may interfere with the formation of disulfide bonds, leading to misfolded proinsulin.
Such misfolded proteins are often nonfunctional and can accumulate in the ER, triggering ER stress.
Over time, prolonged ER stress can impair beta-cell function and even induce apoptosis, reducing insulin production capacity.
Role of Proinsulin Processing in Beta Cells:
After folding, proinsulin undergoes enzymatic cleavage to become mature insulin.
This cleavage is performed by specialized enzymes in the ER, such as prohormone convertases and carboxypeptidase E, which remove specific segments of proinsulin.
Mature insulin is then packaged into secretory vesicles and stored for release in response to elevated glucose levels.
Mutations in the INS gene disrupt this precise sequence. Faulty proinsulin molecules may fail to cleave correctly or may never reach the stage of maturation.
The result is a reduction in functional insulin available for secretion, leading to persistent hyperglycemia.
This impaired processing, coupled with beta-cell dysfunction, is a hallmark of permanent neonatal diabetes mellitus (PNDM) in individuals with INS gene mutations.
How Mutations in the INS Gene Impair Insulin Production?
Here is how it all happens:
Missense Mutations and Protein Misfolding:
Missense mutations result from a single nucleotide change that substitutes one amino acid for another. For example, the R89H mutation alters the amino acid sequence of proinsulin, disrupting its ability to fold properly. Misfolded proinsulin accumulates in the ER, triggering ER stress and impairing beta-cell function.
A study in The Journal of Clinical Investigation (Edghill et al., 2008) demonstrated that such mutations lead to reduced insulin secretion and beta-cell apoptosis, directly contributing to hyperglycemia in neonates.
Frameshift Mutations and Truncated Insulin:
Frameshift mutations involve insertions or deletions of nucleotides, altering the reading frame of the INS gene. These mutations result in truncated proinsulin molecules that are either nonfunctional or toxic to beta cells. For instance, the G32fsdelC mutation has been shown to produce abnormal insulin proteins that exacerbate ER stress and impair glucose regulation.
Research published in Human Mutation (Molven et al., 2008) highlights how frameshift mutations significantly reduce the efficiency of insulin production in neonates, leading to severe PNDM.
Dominant-Negative Mutations and Beta-Cell Stress:
Dominant-negative mutations are particularly severe because they interfere with the function of normal insulin produced by the unaffected allele. These mutations amplify ER stress, leading to widespread beta-cell dysfunction. Affected neonates often present with severe hyperglycemia shortly after birth, requiring intensive management.
Two Live Examples You Need to Read
Let me walk you through these 2 live examples in brief:
Emma’s Missense Mutation as a Newborn:
Emma, a three-month-old infant, was brought to the clinic with symptoms of persistent hyperglycemia, poor weight gain, and frequent dehydration episodes, indicative of neonatal diabetes.
After initial treatment with insulin injections, further investigation was pursued to identify the root cause. Genetic testing revealed a missense mutation in the INS gene—specifically the R89C mutation.
This mutation altered a key amino acid in proinsulin, disrupting the formation of disulfide bonds, which are essential for stabilizing the protein’s structure.
Misfolded proinsulin accumulated in the endoplasmic reticulum (ER), triggering ER stress and subsequent beta-cell apoptosis.
This significantly reduced Emma’s capacity to produce insulin, resulting in her hyperglycemic state.
Thanks to an early diagnosis, Emma’s treatment shifted from conventional insulin injections to sulfonylureas, a class of medications that stimulate remaining beta cells to release insulin.
Within weeks, her blood glucose levels stabilized, her weight normalized, and her quality of life improved.
Emma’s case underscores the transformative power of early genetic testing, allowing for personalized treatment strategies that enhance patient outcomes in neonatal diabetes.
Familial INS Gene Mutations:
In a family with a history of diabetes, two siblings exhibited symptoms of permanent neonatal diabetes mellitus (PNDM), later traced to a frameshift mutation (G32fsdelC) in the INS gene.
The elder sibling, diagnosed at six months, experienced delayed treatment, leading to complications such as growth delays and frequent hospitalizations.
Learning from this experience, the parents opted for genetic screening during the mother’s subsequent pregnancy.
The results confirmed that the second child had inherited the same mutation, enabling immediate postnatal intervention with a tailored treatment plan.
Early initiation of sulfonylurea therapy minimized complications, allowing the younger sibling to thrive without significant setbacks.
This family’s experience highlights the importance of understanding familial patterns of INS gene mutations.
Proactive genetic screening and early intervention can significantly improve outcomes, demonstrating how a familial approach to managing neonatal diabetes can mitigate complications and enhance quality of life.
FAQs on INS Gene and Neonatal Diabetes
Q1: How do mutations in the INS gene lead to neonatal diabetes?
A1: Mutations in the INS gene, which encodes insulin, can disrupt the normal folding and processing of proinsulin. This misfolding leads to the accumulation of unfolded proteins in the endoplasmic reticulum (ER), causing ER stress. The stress response can impair insulin secretion and even result in beta-cell apoptosis, contributing to neonatal diabetes.
Q2: What are the specific effects of INS gene mutations on insulin biosynthesis?
A2: INS gene mutations can decrease insulin biosynthesis through various mechanisms:
Translation Initiation Disruption: Mutations at the translation initiation codon can prevent the synthesis of preproinsulin.
mRNA Stability Reduction: Alterations in the 3′ untranslated region (UTR) can destabilize insulin mRNA, leading to reduced insulin production.
Transcriptional Impairment: Mutations in promoter regions can decrease the transcription of the INS gene, resulting in lower insulin levels.
Q3: How do INS gene mutations affect proinsulin processing?
A3: Certain mutations in the INS gene can lead to the production of proinsulin molecules that are misfolded or improperly processed. This misprocessing can cause the accumulation of proinsulin intermediates, which may not be efficiently converted to active insulin. The accumulation of these intermediates can further exacerbate ER stress and impair insulin secretion.
Q4: Are there specific INS gene mutations associated with neonatal diabetes?
A4: Yes, several mutations in the INS gene have been linked to neonatal diabetes. For example, mutations that alter the proinsulin molecule’s structure can prevent proper folding and processing, leading to beta-cell dysfunction. Additionally, mutations affecting the promoter region can reduce gene expression, resulting in insufficient insulin production.
Q5: How do INS gene mutations impact beta-cell function in neonates?
A5: INS gene mutations can impair beta-cell function by disrupting insulin synthesis and secretion. The accumulation of misfolded proinsulin can induce ER stress, leading to beta-cell apoptosis. Furthermore, these mutations can cause beta-cell dedifferentiation, characterized by the loss of insulin expression and the acquisition of a less differentiated state, further compromising insulin production and secretion.
Conclusion
Mutations in the INS gene disrupt the processes of insulin production, folding, and secretion, leading to neonatal diabetes.
These mutations induce ER stress, impair beta-cell function, and exacerbate hyperglycemia.
Studies published in journals like Diabetologia and Human Mutation underscore the critical role of the INS gene in glucose metabolism and its impact on neonatal health.
Real-life cases, such as Emma’s successful management with sulfonylureas and the siblings diagnosed with familial INS mutations, emphasize the value of early genetic diagnosis and intervention.
While INS mutations cannot be reversed, understanding their mechanisms offers valuable insights into potential therapeutic strategies, paving the way for improved outcomes for neonates with diabetes.
References: