How Hyperinsulinemia Dictates the Onset of Alström Syndrome?
- admin
- January 22, 2025
- 4:58 pm
- No Comments
Alström Syndrome (AS) is a rare genetic disorder that affects multiple organ systems, including the cardiovascular, metabolic, and endocrine systems. Alström Syndrome is also known to damage your liver.
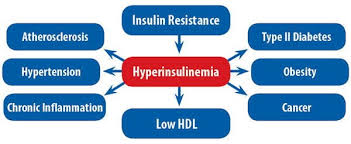
One of the most significant metabolic abnormalities associated with AS is hyperinsulinemia, which plays a critical role in disease progression.
Hyperinsulinemia often precedes insulin resistance and type 2 diabetes mellitus (T2DM), contributing to the overall burden of the syndrome.
Understanding the connection between hyperinsulinemia and AS is essential for improving diagnosis, management, and treatment strategies.
In This Article:
- Overview of Alström Syndrome
- Understanding Hyperinsulinemia
- Genetic Link Between Alström Syndrome and Hyperinsulinemia
- Mechanisms of Hyperinsulinemia in AS
- Clinical Implications of Hyperinsulinemia
- Management Approaches
- FAQs on Hyperinsulinemia and Alström Syndrome
- Conclusion
Overview of Alström Syndrome
Alström Syndrome is an autosomal recessive disorder caused by mutations in the ALMS1 gene, which is located on chromosome 2p13. This gene is essential for intracellular signaling and ciliary function.
According to research published in the Journal of Endocrinological Investigation, ALMS1 mutations disrupt cellular processes, leading to metabolic dysfunction, including insulin resistance and hyperinsulinemia. Patients with AS experience progressive visual and hearing impairments, cardiomyopathy, obesity, and metabolic syndrome.
Studies have shown that metabolic dysfunction is one of the earliest symptoms of AS, with hyperinsulinemia often being detected in childhood before other clinical symptoms become evident.
It is important to make note of the fact that obesity tends to accelerate Alstrom Syndrome.
As per the Journal of Medical Genetics, early diagnosis and intervention are crucial in managing the metabolic complications associated with AS.
Understanding Hyperinsulinemia
Hyperinsulinemia is defined as an excessive concentration of insulin in the bloodstream, typically in response to insulin resistance.
According to Diabetes Care, the pancreas compensates for insulin resistance by producing more insulin to maintain normal glucose levels.
However, chronic hyperinsulinemia can lead to metabolic complications, including impaired glucose tolerance, increased fat storage, and organ damage.
In AS, the overproduction of insulin is an early sign of metabolic dysfunction. Research in the International Journal of Pediatric Endocrinology highlights that AS patients often exhibit fasting hyperinsulinemia even before developing overt diabetes, making it a key diagnostic indicator.
Genetic Link Between Alström Syndrome and Hyperinsulinemia
Mutations in the ALMS1 gene contribute to metabolic disturbances by disrupting intracellular transport and ciliary function.
According to a study in Human Molecular Genetics, the ALMS1 protein plays a crucial role in energy homeostasis and insulin signaling pathways. Dysfunction in these processes leads to impaired glucose uptake by peripheral tissues, promoting insulin resistance and compensatory hyperinsulinemia.
Genetic studies have revealed that patients with AS demonstrate defective insulin signaling pathways, particularly in adipose and muscle tissues, exacerbating hyperinsulinemia.
The European Journal of Human Genetics reports that understanding the genetic basis of AS helps in identifying targeted therapeutic interventions aimed at mitigating insulin dysregulation.
Mechanisms of Hyperinsulinemia in AS
The mechanisms underlying hyperinsulinemia in AS are complex and multifactorial. Some of the key contributing factors include:
- Insulin Resistance: As per Endocrine Reviews, individuals with AS exhibit marked insulin resistance, particularly in skeletal muscle and liver tissues. This resistance forces pancreatic β-cells to overproduce insulin, resulting in hyperinsulinemia.
- Dysfunctional Adipose Tissue: According to Obesity Reviews, adipose tissue dysfunction in AS patients leads to abnormal lipid accumulation and inflammation, further impairing insulin sensitivity and exacerbating hyperinsulinemia.
- Ciliary Dysfunction: The Journal of Clinical Investigation indicates that cilia play a critical role in cellular signaling, and their dysfunction in AS affects insulin receptor function, contributing to metabolic derangements.
- Mitochondrial Dysfunction: Studies in Diabetes & Metabolism Journal suggest that mitochondrial abnormalities in AS patients lead to impaired energy metabolism, further driving insulin resistance and hyperinsulinemia.
Clinical Implications of Hyperinsulinemia
Hyperinsulinemia in Alström Syndrome has significant clinical implications, influencing the progression and severity of various complications. Some of the key consequences include:
- Early-Onset Type 2 Diabetes Mellitus: Chronic hyperinsulinemia eventually leads to pancreatic β-cell exhaustion, resulting in insulin deficiency and the onset of Type 2 Diabetes. According to The Lancet Diabetes & Endocrinology, the transition from hyperinsulinemia to diabetes in AS patients often occurs rapidly, necessitating early intervention.
- Cardiovascular Risks: Elevated insulin levels contribute to hypertension, dyslipidemia, and atherosclerosis, increasing the risk of cardiovascular diseases. The American Journal of Cardiology highlights that individuals with AS are at a higher risk of developing cardiomyopathy due to prolonged hyperinsulinemia.
- Hepatic Involvement: Studies in Hepatology International suggest that hyperinsulinemia contributes to the development of non-alcoholic fatty liver disease (NAFLD) in AS patients, leading to hepatic fibrosis and cirrhosis.
- Renal Complications: Persistent hyperinsulinemia has been linked to glomerular hyperfiltration and albuminuria, which may accelerate the progression of chronic kidney disease in AS patients, as reported in Nephrology Dialysis Transplantation.
Management Approaches
Effective management of hyperinsulinemia in AS requires a comprehensive approach that includes lifestyle interventions, pharmacotherapy, and regular monitoring.
- Lifestyle Modifications: As per The Journal of Clinical Endocrinology & Metabolism, dietary interventions focusing on low glycemic index foods and increased physical activity can help improve insulin sensitivity and reduce hyperinsulinemia.
- Pharmacological Interventions: Medications such as metformin and thiazolidinediones have been shown to enhance insulin sensitivity in AS patients. The International Journal of Obesity reports that metformin therapy can effectively lower insulin levels and improve metabolic outcomes in AS.
- Regular Monitoring: Continuous glucose monitoring (CGM) and periodic insulin level assessments are crucial for tracking disease progression and adjusting treatment strategies. According to Diabetes Technology & Therapeutics, CGM provides real-time data that helps optimize metabolic control in AS patients.
- Emerging Therapies: Research in Nature Metabolism suggests that novel treatments targeting insulin signaling pathways and mitochondrial function may offer promising results for managing hyperinsulinemia in AS.
FAQs on Hyperinsulinemia and Alström Syndrome
Q-1: How does hyperinsulinemia influence the early metabolic disturbances seen in Alström Syndrome?
A-1: Hyperinsulinemia, an excess of insulin in the blood, plays a critical role in the early metabolic disturbances characteristic of Alström Syndrome. It acts as a compensatory response to insulin resistance, a hallmark of the syndrome. This elevated insulin level attempts to maintain normal blood glucose by forcing glucose uptake into cells.
However, persistent hyperinsulinemia exacerbates cellular stress and disrupts normal metabolic signaling pathways. In Alström Syndrome, this contributes to early onset obesity, dyslipidemia, and impaired glucose tolerance, setting the stage for progressive diabetes and other metabolic complications.
Q-2: Can hyperinsulinemia accelerate the progression of organ dysfunction in Alström Syndrome?
A-2: Yes, hyperinsulinemia can accelerate organ dysfunction in Alström Syndrome by promoting systemic inflammation and fibrosis. High insulin levels affect multiple organ systems, including the heart, liver, and kidneys, which are already vulnerable due to the genetic mutations underlying the syndrome.
Hyperinsulinemia-driven pathways induce fibrotic changes and tissue remodeling, worsening cardiomyopathy, hepatic steatosis, and renal impairment. This exacerbation contributes to the multi-organ decline that defines the severity of Alström Syndrome.
Q-3: How does hyperinsulinemia interact with genetic factors to dictate the phenotypic variability in Alström Syndrome?
A-3: Hyperinsulinemia’s interaction with the ALMS1 gene mutation influences the phenotypic variability observed in Alström Syndrome. While the mutation causes ciliary dysfunction affecting cellular metabolism, hyperinsulinemia acts as a modifier that intensifies metabolic dysfunction.
Variations in insulin sensitivity and secretion among individuals can lead to differing degrees of metabolic symptoms and disease severity. Thus, hyperinsulinemia doesn’t act in isolation but amplifies the genetic defect’s impact, dictating a spectrum of clinical presentations.
Q-4: Is hyperinsulinemia a potential therapeutic target to delay or mitigate Alström Syndrome progression?
Interventions such as insulin sensitizers, lifestyle modification, and dietary management could potentially modulate hyperinsulinemia’s adverse effects, improving quality of life and delaying complications associated with the syndrome.
Q-5: What role does hyperinsulinemia play in the neurological symptoms associated with Alström Syndrome?
A-5: Hyperinsulinemia may contribute to neurological symptoms in Alström Syndrome through its impact on neuronal metabolism and inflammation. Excess insulin can alter brain glucose metabolism, potentially leading to cognitive impairment and neurodegeneration.
Additionally, insulin’s role in promoting systemic inflammation could exacerbate neuroinflammatory processes. As per bestdietarysupplementfordiabetics.com, “While less studied, these mechanisms suggest that hyperinsulinemia is not only a metabolic concern but also a factor influencing the neurological manifestations of the syndrome”.
Conclusion
Hyperinsulinemia plays a pivotal role in the onset and progression of Alström Syndrome, serving as an early indicator of metabolic dysfunction.
Elevated insulin levels contribute to the development of T2DM, cardiovascular diseases, and multi-organ complications in AS patients.
Understanding the genetic and physiological mechanisms underlying hyperinsulinemia is crucial for developing targeted treatment strategies.
Early detection, lifestyle modifications, and pharmacological interventions remain key to managing hyperinsulinemia and improving the quality of life for individuals with Alström Syndrome.
Continued research and advancements in precision medicine hold the potential to address the metabolic challenges associated with AS effectively.
References:
