How Inheritance Impacts the Genetic Basis of MODY Diabetes?
- admin
- November 27, 2024
- 7:58 pm
- No Comments

In this article, BestDietarySupplementforDiabetics will explore the intricate relationship between inheritance and the genetic basis of MODY diabetes.
Topics will include the role of specific genes, inheritance patterns, and real-life examples to illustrate how genetic factors contribute to MODY.
This Article Covers:
- Introduction to MODY Diabetes
- Autosomal Dominant Inheritance and MODY
- Key Genes Involved in MODY Development
- How Inherited Mutations Impact Beta-Cell Function
- Environmental and Lifestyle Interactions
- Real-Life Example: Anna’s MODY Diagnosis
- FAQs on Inheritance and MODY Diabetes
- Genetic Counseling for MODY Families
- Conclusion: Understanding Inheritance in MODY
Introduction to MODY Diabetes
Maturity-Onset Diabetes of the Young (MODY) is a rare, monogenic form of diabetes, often diagnosed in adolescents or young adults.
Unlike Type 1 or Type 2 diabetes, MODY stems from mutations in a single gene responsible for regulating pancreatic beta-cell function and insulin secretion.
These genetic mutations disrupt normal glucose metabolism, leading to persistent hyperglycemia.
What makes MODY particularly unique is its autosomal dominant inheritance pattern, meaning it is passed down from one generation to the next.
Its genetic origins highlight the critical role of inheritance in disease development, making MODY an essential focus for understanding monogenic diabetes disorders.
Autosomal Dominant Inheritance and MODY
One defining feature of Maturity-Onset Diabetes of the Young (MODY) is its autosomal dominant inheritance pattern, meaning an affected individual has a 50% chance of passing the condition to their offspring.
This genetic mechanism underpins why MODY frequently runs in families.
Key Characteristics of MODY Inheritance:
- Single Gene Mutations: MODY is caused by mutations in specific genes, such as HNF1A or GCK, unlike Type 2 diabetes, which results from multiple genetic and environmental factors.
- Generational Impact: Its autosomal dominant pattern ensures consistent appearance across generations, often leading to early diagnoses within family lineages.
- Defective Insulin Secretion: MODY stems from impaired insulin secretion rather than insulin resistance, setting it apart from other diabetes types.
A study in Diabetologia emphasizes that families with a history of diabetes diagnosed before age 25 should be screened for MODY, highlighting the central role inheritance plays in its occurrence and early identification.
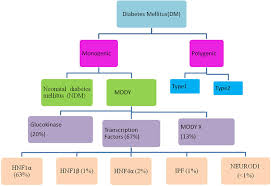
Key Genes Involved in MODY Development
Maturity-Onset Diabetes of the Young (MODY) arises from mutations in at least 14 identified genes, with three being the most prevalent:
- HNF1A (MODY3): Mutations in this gene impair pancreatic beta-cell function, reducing insulin secretion and leading to glucose intolerance. As the most common MODY subtype, MODY3 accounts for nearly 50% of all cases.
- GCK (MODY2): The glucokinase (GCK) gene plays a key role in glucose sensing. Mutations result in mild, stable hyperglycemia that often does not progress or require aggressive treatment.
- HNF4A (MODY1): This gene influences insulin production and secretion. Mutations disrupt these processes, causing a more progressive form of diabetes over time.
These genes illustrate the critical role of genetic mutations in beta-cell dysfunction and insulin regulation.
For example, studies published in The Journal of Clinical Endocrinology & Metabolism demonstrate how mutations in these genes impair glucose sensing and insulin secretion pathways, making MODY distinct from other diabetes types.
Understanding these key genes is crucial for accurate diagnosis and tailored treatment strategies.
How Inherited Mutations Impact Beta-Cell Function?
Inherited mutations associated with MODY diabetes directly impair the functionality of pancreatic beta cells, the insulin-producing units responsible for glucose regulation.
Here is how these mutations exert their effects:
Impaired Glucose Sensing:
Mutations in the GCK gene hinder the ability of beta cells to detect blood glucose levels accurately.
This leads to a failure in triggering adequate insulin release, causing persistent, mild hyperglycemia.
However, unlike other forms of diabetes, severe complications are uncommon in MODY2, which is GCK-related.
Reduced Insulin Production:
Mutations in transcription factor genes like HNF1A and HNF4A disrupt the regulatory pathways necessary for insulin gene expression.
This results in insufficient insulin production, even when glucose levels are elevated, exacerbating blood sugar imbalances.
Progressive Beta-Cell Decline:
Inherited mutations lead to a gradual loss of beta-cell efficiency over time.
Unlike the autoimmune destruction seen in Type 1 diabetes, MODY-related progression stems from genetic defects in beta-cell functionality rather than immune-mediated attacks.
Research Evidence:
A study published in the Journal of Clinical Endocrinology & Metabolism revealed that patients with MODY3, caused by HNF1A mutations, experience a 30% decline in beta-cell function per decade, highlighting the long-term impact of these inherited genetic mutations.
This progressive decline underscores the importance of early diagnosis and tailored treatments to slow disease progression and preserve remaining beta-cell function.
Environmental and Lifestyle Interactions
While MODY is fundamentally genetic, environmental and lifestyle factors significantly influence its progression and symptom severity.
Here is how:
- Dietary Habits: High-sugar diets can worsen glucose dysregulation, particularly in MODY2 patients with impaired glucose sensing. This can lead to higher fasting blood glucose levels and increased long-term complications.
- Physical Activity: A sedentary lifestyle accelerates beta-cell dysfunction in individuals with genetic predispositions, further reducing insulin secretion efficiency. Exercise, on the other hand, enhances glucose uptake and supports overall metabolic health.
- Chronic Stress: Stress hormones like cortisol can exacerbate glucose variability, making blood sugar levels harder to manage for those with MODY.
By understanding these interactions, individuals with MODY can adopt tailored lifestyle strategies to complement medical management.
For example, a study in Diabetologia highlights how combining dietary modifications with regular exercise significantly improves glucose control in MODY patients, underscoring the importance of lifestyle factors.
Anna’s MODY Diagnosis:
Anna, a 22-year-old college student, experienced a life-changing moment when a routine health screening revealed fasting hyperglycemia.
Without typical markers of insulin resistance or autoimmunity, her endocrinologist suspected a monogenic form of diabetes—MODY.
Genetic testing later confirmed an HNF1A gene mutation, diagnosing Anna with MODY3, the most common subtype.
Initially, Anna had been misdiagnosed with Type 1 diabetes and prescribed insulin therapy, which caused frequent hypoglycemic episodes and frustration.
Upon receiving her MODY diagnosis, her treatment was adjusted to sulfonylureas, which effectively stimulated her insulin production and brought her blood sugar under control.
Anna’s journey highlights the critical role of genetic testing in differentiating MODY from other diabetes forms.
Early identification not only simplifies treatment but also spares patients from unnecessary and invasive therapies.
Anna’s experience underscores the importance of considering genetic factors in diabetes diagnosis and management.
FAQs on Inheritance & MODY Diabetes
Q-1: Why does the inheritance pattern make MODY show up “earlier” in some families than others?
A-1: Most MODY genes are autosomal dominant, so a single altered copy can express at any age—but when it crosses the diagnostic threshold depends on life-stage stressors.
Puberty, pregnancy, rapid growth, illness, or weight gain can unmask a preexisting secretion defect. That is why one generation presents in their teens, while a parent with the same variant wasn’t labeled until their 30s after pregnancy or midlife weight change.
Q-2: Can who passes down the variant (mother vs. father) change how MODY looks at birth and beyond?
A-2: Yes—parent-of-origin can matter for some subtypes. When a mother with a glucokinase (GCK) variant carries a fetus without the variant, the fetus experiences relatively higher glucose and may grow larger; if the fetus does inherit the variant, growth tends to normalize.
Certain HNF4A families show distinctive neonatal features (e.g., transient hypoglycemia or higher birthweight) that depend on transmission. Same gene, same dominance—different perinatal expression.
Q-3: How can inheritance point to MODY even when gene sequencing is “negative”?
A-3: A clear vertical pattern (diabetes in successive generations, early onset, lean body habitus, preserved C-peptide, negative autoantibodies) can signal MODY mechanisms that basic panels miss: copy-number changes deleting an entire gene, regulatory variants silencing enhancers/promoters, or parental mosaicism where a parent carries the variant in some germ cells but not blood. In those families, adding copy-number/structural testing or re-testing over time is often decisive.
Q-4: Why do siblings with the same dominant MODY variant need different treatments?
A-4: Inheritance sets risk, not dose. Modifier genes (affecting β-cell stress, lipid handling, incretin biology), fetal environment, and lifestyle tilt expression.
One HNF1A carrier may thrive on very low-dose sulfonylurea; another, with identical DNA change, needs combination therapy during hormonally demanding phases (puberty, pregnancy). Polygenic background can also make a “pure” monogenic picture look more insulin-resistant or, conversely, milder than expected.
Q-5: How does understanding inheritance change family testing and day-to-day care?
A-5: A confirmed MODY variant turns the family tree into a roadmap: each first-degree relative has ~50% chance of carrying it, so targeted cascade testing is efficient.
A precise label guides therapy (e.g., sulfonylurea sensitivity in many HNF1A/HNF4A cases; minimal pharmacotherapy in most GCK outside pregnancy) and focuses surveillance where it counts (e.g., kidney and electrolyte checks in HNF1B).
Correct classification prevents years of mislabeling as Type 1 or 2 and aligns treatment with the underlying biology.
Genetic Counseling for MODY Families
Given the inherited nature of MODY, genetic counseling plays an essential role in managing this monogenic form of diabetes.
Counseling offers comprehensive support to families by addressing several key areas:
Risk Assessment:
Genetic counselors analyze family medical histories to determine which relatives are at risk of inheriting MODY.
This is especially relevant given MODY’s autosomal dominant inheritance pattern, where there is a 50% chance of passing the mutation to offspring.
Diagnostic Strategies:
Counselors assist in selecting and interpreting genetic tests to confirm MODY.
Identifying the specific mutation, such as in the HNF1A, HNF4A, or GCK genes, ensures accurate diagnosis and optimal treatment plans for affected individuals.
Reproductive Planning:
Counseling provides prospective parents with crucial information about the likelihood of passing MODY to their children.
This empowers families to make informed reproductive decisions and consider options like early genetic screening for their offspring.
Case Study in Counseling:
A three-generation family with early-onset diabetes sought genetic counseling. Testing revealed an HNF4A mutation, confirming MODY1 in affected members.
This led to tailored treatments, improved glycemic control, and early interventions for at-risk children, preventing complications and providing peace of mind for the family.
By bridging genetic science with patient care, genetic counseling ensures that MODY families are equipped with the knowledge to manage and mitigate the condition effectively.
Takeaway: Understanding Inheritance in MODY
Inheritance is the cornerstone of MODY’s genetic basis, influencing its diagnosis, progression, and management.
By understanding how autosomal dominant patterns affect beta-cell function and insulin production, we can better identify and treat this rare form of diabetes.
Genetic testing, counseling, and tailored interventions remain essential for families navigating the challenges of MODY.
Through continued research, we can further uncover the complexities of inheritance in MODY and improve outcomes for affected individuals.
References: